Proiect PN-II-ID-PCE-2012-4-0248:
Project timespan
Project team
Abstract
Project objectives
Budget
Results
Title: Innovative Mechanically Interlocked Molecules: Design, Synthesis, Properties and Molecular Devices
Project manager: Prof. Dr. Ion Grosu ResearcherID: B-8088-2011
Team:
- Dr. Elena Bogdan ResearcherID: F-1379-2011
- Dr. Niculina D. Hădade ResearcherID: F-9489-2011
- Dr. Anamaria Terec ResearcherID: C-2571-2011
- PhD Cosmin Crişan
- PhD Lakatos Eszter
- MSc Cristian Victor Reţe
- MSc Ana-Maria Casian
- BSc Traistaru Anamaria
Project timespan
2013 -- 2016
2013 -- 2016
Abstract
The major goals of this project are the synthesis, structural investigations and elaboration of the mechanism of work for molecular devices based on mechanically interlocked molecules exhibiting new cryptand units. The target cryptands show three or four chains and are built up using 2,4,6-triaryl-1,3,5-triazine, tetrasubstituted pyrenes or biphenyls as caps and disubstituted 1,10-phenantrolyne or 2,2'-bipyridine units as chains. The positions of the heterocyclic units in the chains allow the complexation of other similar heterocyclic derivatives with Cu+, Ag+,Zn2+ either inside (for IN-chains) or outside (for OUT chains) the cavity of the cryptand. The connection of the guest complexed heterocyclic units ones to the others in the all-IN cryptands leads to the formation of interlocked entities having macrocycles entrapped in the cavity of the cryptands. The same bonding with the outside guests (all OUT cryptands) form entities with cryptands entrapped in the cavity of the macrocycles (they are [2]rotaxanes having the cryptand as axle). For the cryptands with mixed chains (IN and OUT chains) the formation of the macrocycles with the guest units leads to [2]cryptocatenands or knotted [2]cryptocatenands [interlocked cryptands with (knotted) macrocycles]. The macrocycles can rotate (either inside or outside the cryptand) or can wind around the chains, the motion of the macrocycles can be chemically or electrochemically controlled and the target compounds are molecular ROTORS.
The major goals of this project are the synthesis, structural investigations and elaboration of the mechanism of work for molecular devices based on mechanically interlocked molecules exhibiting new cryptand units. The target cryptands show three or four chains and are built up using 2,4,6-triaryl-1,3,5-triazine, tetrasubstituted pyrenes or biphenyls as caps and disubstituted 1,10-phenantrolyne or 2,2'-bipyridine units as chains. The positions of the heterocyclic units in the chains allow the complexation of other similar heterocyclic derivatives with Cu+, Ag+,Zn2+ either inside (for IN-chains) or outside (for OUT chains) the cavity of the cryptand. The connection of the guest complexed heterocyclic units ones to the others in the all-IN cryptands leads to the formation of interlocked entities having macrocycles entrapped in the cavity of the cryptands. The same bonding with the outside guests (all OUT cryptands) form entities with cryptands entrapped in the cavity of the macrocycles (they are [2]rotaxanes having the cryptand as axle). For the cryptands with mixed chains (IN and OUT chains) the formation of the macrocycles with the guest units leads to [2]cryptocatenands or knotted [2]cryptocatenands [interlocked cryptands with (knotted) macrocycles]. The macrocycles can rotate (either inside or outside the cryptand) or can wind around the chains, the motion of the macrocycles can be chemically or electrochemically controlled and the target compounds are molecular ROTORS.
Objectives
The major goals of this project are the synthesis, structural investigations and elaboration of the mechanism of work for molecular devices based on mechanically interlocked molecules exhibiting new cryptand units (Chart 3). The target molecules are new supramolecular assemblies, unprecedented in the literature and consist in cryptand-macrocycles supramolecular assemblies (I and II), original (pseudo)[2]rotaxanes (III and IV), macrocycle-cryptand catenanes (V and VI) and a new type of mechanically interlocked molecules (accordingly with the recent review of Leigh) for which we propose the name of knotted [2]cryptocatenane (VII). In I and II for the first time, macrocycles are entrapped in the cavities of cryptands, III and IV are the first rotaxanes in which the axle is a cryptand, V and VI are catenanes with interlocked macrocycles and cryptands and VII is a catenane formed by the interlocking of a cryptand with a winding macrocycle.
Targets I and II (V and VI) can exhibit a pirouetting of the macrocycles inside the cavity of the cryptand (or around one or two chains of the cryptand), while in III and IV the macrocycle can rotate around the cryptand. For VII a winding rotation of the macrocycle around the chains of the cryptand can be predicted. Target compounds I-VII are molecular ROTORS (SWITCHES) and the movements of the different units can be chemically or electrochemically controlled.
O1. Synthesis and structural investigation of new mechanically interlocked supramolecular systems: macrocycles incarcerated in cryptands (I and II), (pseudo)rotaxanes (III and IV), cryptocatenanes (V and VI) and knotten cryptocatenanes (VII) (Chart 1);
O2. Investigations of the technomimetic properties of I-VII and building of chemically and electrochemically controlled molecular devices;
O3. Management of the project, formation of young researches, development of the infrastructure and dissemination of the results.
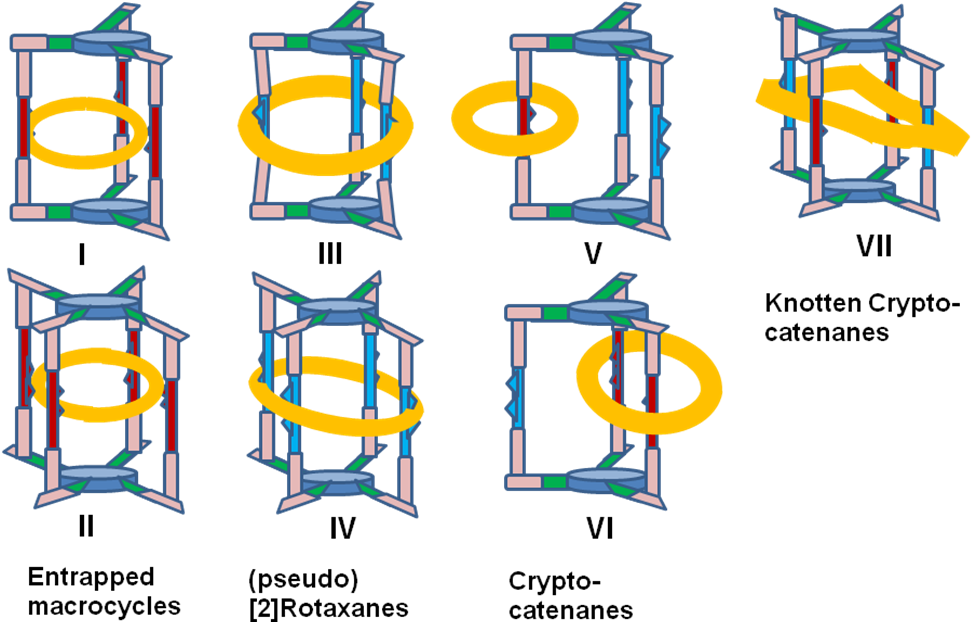
Chart 1. Target cryptands based mechanically interlocked supramolecules.
Tasks (Tn):
T1. Management of the project. Correct planning and verification of activities, the planning of resources, the analysis of the feed-back, the ensuring of the dynamics of the research. T2. Obtaining of the precursors of the target cryptands: central units, chains, spacers.
T3. Synthesis and structural analysis of All-IN and All-OUT cryptands.
T4. Synthesis and structural analysis of cryptands with entrapped macrocycles (I, II; Chart 3) and cryptands having macrocycles as belts.
T5. Synthesis and structural analysis of cryptands with different chains (IX, X and XIV; Chart 4) and of the corresponding cryptocatenanes and knotted catenanes.
T6. Investigations of the molecular device properties of compounds I-VII. Feed-back on the design of target compounds. Modifications (minor) of the target compounds in order to get new interlocked compounds with improved technomimetic properties.
T7. Young scientists formation (YR), improvement of the scientific environment (INF) and dissemination (DIS) of the results. These aims will be carried out by group seminars and meetings (YR), invited lectures (YR), periodic reports (YR), acquisition of equipments (INF), participation to congresses and writing of the papers and PhD Theses (DIS).
The major goals of this project are the synthesis, structural investigations and elaboration of the mechanism of work for molecular devices based on mechanically interlocked molecules exhibiting new cryptand units (Chart 3). The target molecules are new supramolecular assemblies, unprecedented in the literature and consist in cryptand-macrocycles supramolecular assemblies (I and II), original (pseudo)[2]rotaxanes (III and IV), macrocycle-cryptand catenanes (V and VI) and a new type of mechanically interlocked molecules (accordingly with the recent review of Leigh) for which we propose the name of knotted [2]cryptocatenane (VII). In I and II for the first time, macrocycles are entrapped in the cavities of cryptands, III and IV are the first rotaxanes in which the axle is a cryptand, V and VI are catenanes with interlocked macrocycles and cryptands and VII is a catenane formed by the interlocking of a cryptand with a winding macrocycle.
Targets I and II (V and VI) can exhibit a pirouetting of the macrocycles inside the cavity of the cryptand (or around one or two chains of the cryptand), while in III and IV the macrocycle can rotate around the cryptand. For VII a winding rotation of the macrocycle around the chains of the cryptand can be predicted. Target compounds I-VII are molecular ROTORS (SWITCHES) and the movements of the different units can be chemically or electrochemically controlled.
O1. Synthesis and structural investigation of new mechanically interlocked supramolecular systems: macrocycles incarcerated in cryptands (I and II), (pseudo)rotaxanes (III and IV), cryptocatenanes (V and VI) and knotten cryptocatenanes (VII) (Chart 1);
O2. Investigations of the technomimetic properties of I-VII and building of chemically and electrochemically controlled molecular devices;
O3. Management of the project, formation of young researches, development of the infrastructure and dissemination of the results.

Chart 1. Target cryptands based mechanically interlocked supramolecules.
Tasks (Tn):
T1. Management of the project. Correct planning and verification of activities, the planning of resources, the analysis of the feed-back, the ensuring of the dynamics of the research. T2. Obtaining of the precursors of the target cryptands: central units, chains, spacers.
T3. Synthesis and structural analysis of All-IN and All-OUT cryptands.
T4. Synthesis and structural analysis of cryptands with entrapped macrocycles (I, II; Chart 3) and cryptands having macrocycles as belts.
T5. Synthesis and structural analysis of cryptands with different chains (IX, X and XIV; Chart 4) and of the corresponding cryptocatenanes and knotted catenanes.
T6. Investigations of the molecular device properties of compounds I-VII. Feed-back on the design of target compounds. Modifications (minor) of the target compounds in order to get new interlocked compounds with improved technomimetic properties.
T7. Young scientists formation (YR), improvement of the scientific environment (INF) and dissemination (DIS) of the results. These aims will be carried out by group seminars and meetings (YR), invited lectures (YR), periodic reports (YR), acquisition of equipments (INF), participation to congresses and writing of the papers and PhD Theses (DIS).
Budget
| Budget chapter (expenses) | 2013 (lei) | 2014 (lei) | 2015 (lei) | 2015 (lei) | Total | |
|---|---|---|---|---|---|---|
| 1 | Salaries | 100.000 | 200.000 | 140.000 | 100.000 | 540.000 |
| 2 | Overhead | 24.000 | 45.089,74 | 57.391,30 | 39.171,13 | 165.652,17 |
| 3 | Mobility | 0 | 20.000 | 24.000 | 20.347,83 | 64.347,83 |
| 4 | Logistics | 60.000 | 80.598,26 | 218.608,70 | 140.793,04 | 500.000 |
| 5 | Total | 184.000 | 345.688 | 440.000 | 300.312 | 1.270.000 |
Results 2013
The obtaining of the central units was carried out using procedures already presented in the literature or already used in our group.1,2 (Schemes 1 and 2)
The building blocks of the bridges with phenantroline and bipyril units were obtained starting from commercially available compounds as dimethylphenantrolines and dimethylbipyridines. The steps which lead to derivatives with -CHO, CH2OH and CH2Br groups are shown in Schemes 3 and 4.3 These functions allow the macrocyclization reactions with the already obtained central units.
Reactions shown in schemes 1-4 were already carried out and now the purity of the compounds is investigated and optimizations of the syntheses are under consideration.
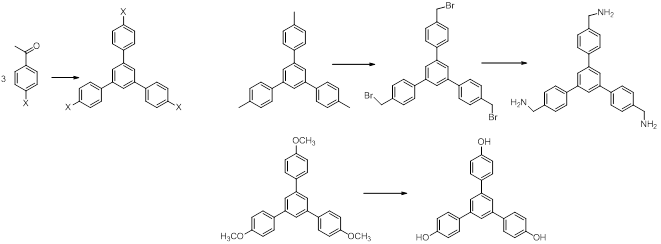
Scheme 1
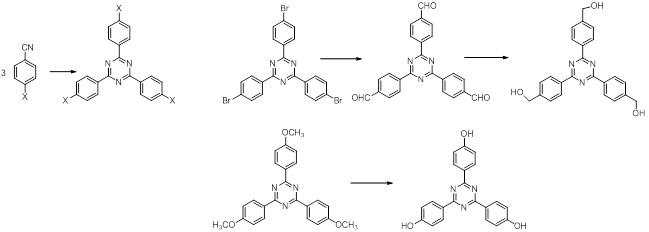
Scheme 2
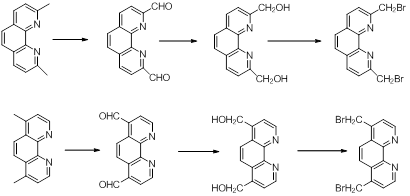
Scheme 3
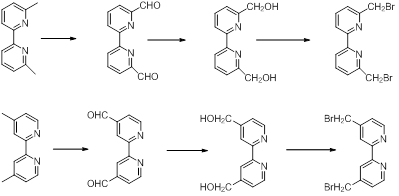
Scheme 4
In paralel, using a cryptand already obtained in our group we carried out investigations concerning the complexation ability of the cryptand for aromatic compounds as guests. The ainm was to elaborate the protocols for similar investigations with target cryptands. The used methods were based on NMR, UV-VIS and CV; the better results being obtained with CV.4
1. a. Mastalerz M., Angew. Chem. Int. Ed., 49, 5042-5053 (2010); b. Barwell N. P., Crump M. P., Davis A. P., Angew. Chem. Int. Ed., 48, 7673-7676 (2009); c. Ferrand Y., Klein E., Barwell N. P., Crump M. P., Barbero J. J., Vincent C., Boons G.-J., Ingale S., Davis A. P., Angew. Chem. Int. Ed., 48, 1775-1779 (2009); d. Atwood J. L., MacGillivray L. R., Angew. Chem. Int. Ed., 38, 1018-1033 (1999).
2. Woiczechowski-Pop, A.; Dobra, I. L.; Roiban, G. D.; Terec, A.; Grosu, I. Synth. Commun., 2012, 42, 3579-3588.
3. a. Alyapyshev M. Y., Babain V. A., Borisova N. E., Kiseleva R. N., Safronov D. V., Reshetova M. D., Mendeleev Communications, 18, 336-337 (2008); b. Kiehne U., Bunzen J., Staats H., Lutzen A. Synthesis, 1061-1069 (2007); c. Ishi-i T., Yaguma K., Kuwahara R., TaguriY., Mataka S. Org. Lett., 8, 585-588 (2006); d. Higashi T., Inami K., Mochizuki M. J. Heterocyclic Chem., 45, 1889-1892 (2008); e. De Cian A., DeLemos E., Mergny J.-L., Teulade-Fichou M.-P., Monchaud D., J. Am. Chem. Soc., 129, 1856-1857 (2007); f. Eggers F., Lüning U. Eur. J. Org. Chem., 2328-2341 (2009).
4. Woiczechowski-Pop, A.; Gligor, D.; Bende, A.; Varodi, C.; Bogdan, E.; Terec, A.; Grosu, I. Synthesis, Structure, Electrochemical Behavior and Electrochemical Investigations on the Assembling with Pyrene of a Novel C3 Cryptand; submitted.
Dissemination
The obtaining of the central units was carried out using procedures already presented in the literature or already used in our group.1,2 (Schemes 1 and 2)
The building blocks of the bridges with phenantroline and bipyril units were obtained starting from commercially available compounds as dimethylphenantrolines and dimethylbipyridines. The steps which lead to derivatives with -CHO, CH2OH and CH2Br groups are shown in Schemes 3 and 4.3 These functions allow the macrocyclization reactions with the already obtained central units.
Reactions shown in schemes 1-4 were already carried out and now the purity of the compounds is investigated and optimizations of the syntheses are under consideration.

Scheme 1

Scheme 2

Scheme 3

Scheme 4
In paralel, using a cryptand already obtained in our group we carried out investigations concerning the complexation ability of the cryptand for aromatic compounds as guests. The ainm was to elaborate the protocols for similar investigations with target cryptands. The used methods were based on NMR, UV-VIS and CV; the better results being obtained with CV.4
1. a. Mastalerz M., Angew. Chem. Int. Ed., 49, 5042-5053 (2010); b. Barwell N. P., Crump M. P., Davis A. P., Angew. Chem. Int. Ed., 48, 7673-7676 (2009); c. Ferrand Y., Klein E., Barwell N. P., Crump M. P., Barbero J. J., Vincent C., Boons G.-J., Ingale S., Davis A. P., Angew. Chem. Int. Ed., 48, 1775-1779 (2009); d. Atwood J. L., MacGillivray L. R., Angew. Chem. Int. Ed., 38, 1018-1033 (1999).
2. Woiczechowski-Pop, A.; Dobra, I. L.; Roiban, G. D.; Terec, A.; Grosu, I. Synth. Commun., 2012, 42, 3579-3588.
3. a. Alyapyshev M. Y., Babain V. A., Borisova N. E., Kiseleva R. N., Safronov D. V., Reshetova M. D., Mendeleev Communications, 18, 336-337 (2008); b. Kiehne U., Bunzen J., Staats H., Lutzen A. Synthesis, 1061-1069 (2007); c. Ishi-i T., Yaguma K., Kuwahara R., TaguriY., Mataka S. Org. Lett., 8, 585-588 (2006); d. Higashi T., Inami K., Mochizuki M. J. Heterocyclic Chem., 45, 1889-1892 (2008); e. De Cian A., DeLemos E., Mergny J.-L., Teulade-Fichou M.-P., Monchaud D., J. Am. Chem. Soc., 129, 1856-1857 (2007); f. Eggers F., Lüning U. Eur. J. Org. Chem., 2328-2341 (2009).
4. Woiczechowski-Pop, A.; Gligor, D.; Bende, A.; Varodi, C.; Bogdan, E.; Terec, A.; Grosu, I. Synthesis, Structure, Electrochemical Behavior and Electrochemical Investigations on the Assembling with Pyrene of a Novel C3 Cryptand; submitted.
Dissemination
- Woiczechowski-Pop, A.; Gligor, D.; Bende, A.; Varodi, C.; Bogdan, E.; Terec, A.; Grosu, I. Synthesis, Structure, Electrochemical Behavior and Electrochemical Investigations on the Assembling with Pyrene of a Novel C3 Cryptand; submitted.
-
Pleanary Lecture (Ion Grosu): Cyclophanes, Cryptands and H-bonding Self-assembled Aggregates: Synthesis, Structure and Molecular Machines; Romanian International Conference on Chemistry and Chemical Engineering, RICCCE 18 Sinaia 3-7 sept. 2013.
Results 2014
In order to determine the reactivity of the target aromatic units, the procedures of macrocyclization and of the requested investigations for the structure determinations some cryptands exhibiting the target aromatic units and having oligoethyleneoxide bridges were obtained in one or two steps accordingly with Scheme 1.
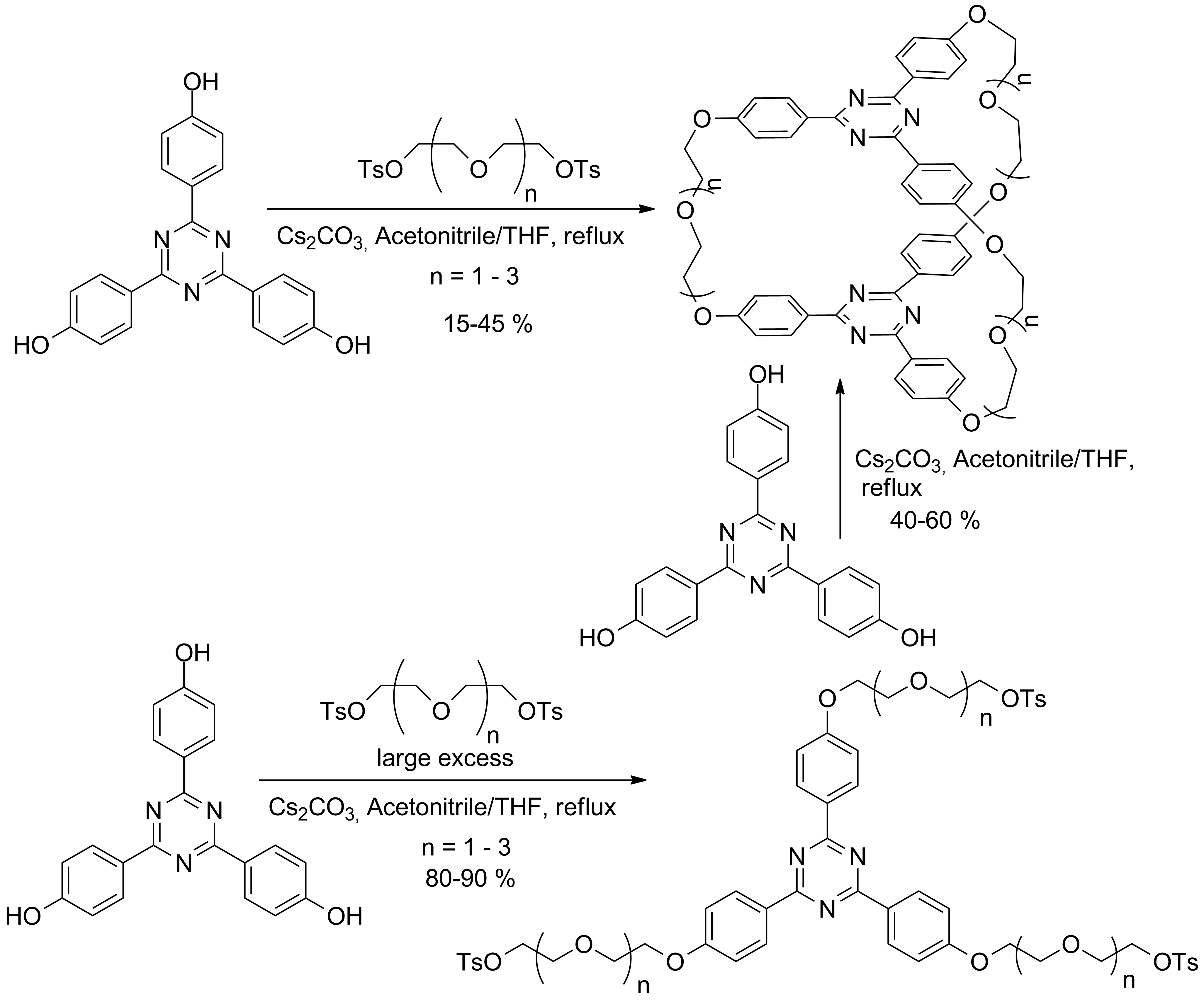
Scheme 1
The structure of the compounds were determined using NMR and MS methods and the complexation ability was investigated using ESI-MS techniques.
For the obtaining of the target interlocked molecules we changed the aromatic reference groups (replacing the triazine unit with a benzene ring) and the bridges (we enlarged the size of the phenantroline derivatives in order to be able to accommodate the target complexes). We decided to use as macrocyclization procedure a metal free click reaction and so we obtained the bridges decorated with azide functions (A and B) and the aromatic reference group was decorated with cyclooctyne moieties (C, Scheme 2).
The click reactions of the azide and cyclooctyne decorated bridges and aromatic reference units lead to the all-in (I) and all-out (II) cryptands in acceptable yields (Scheme 3).
The cryptands were complexed at the phenantroline units with appropriated cations and phenantrolines decorated at positions 2,7 with CH2-N3 groups. The reaction of the complexes with podands having cyclooctyne units at their extremities lead to the desired interlocked molecules. These reactions and products are under investigations.
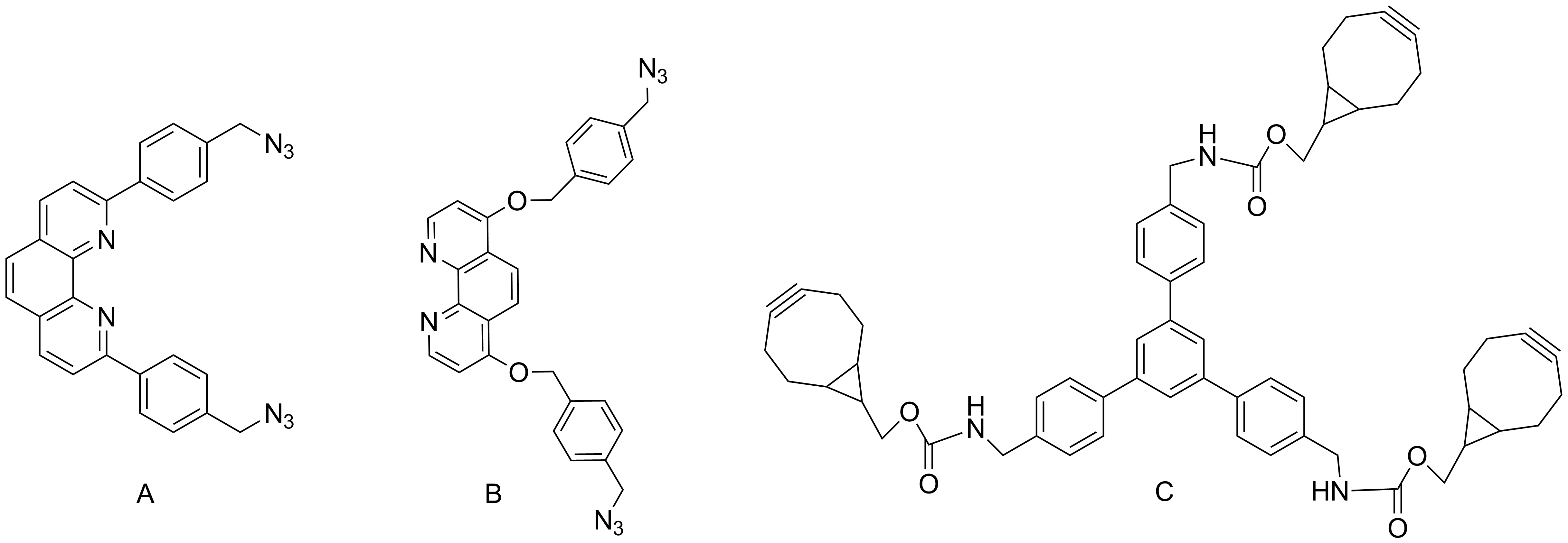
Scheme 2
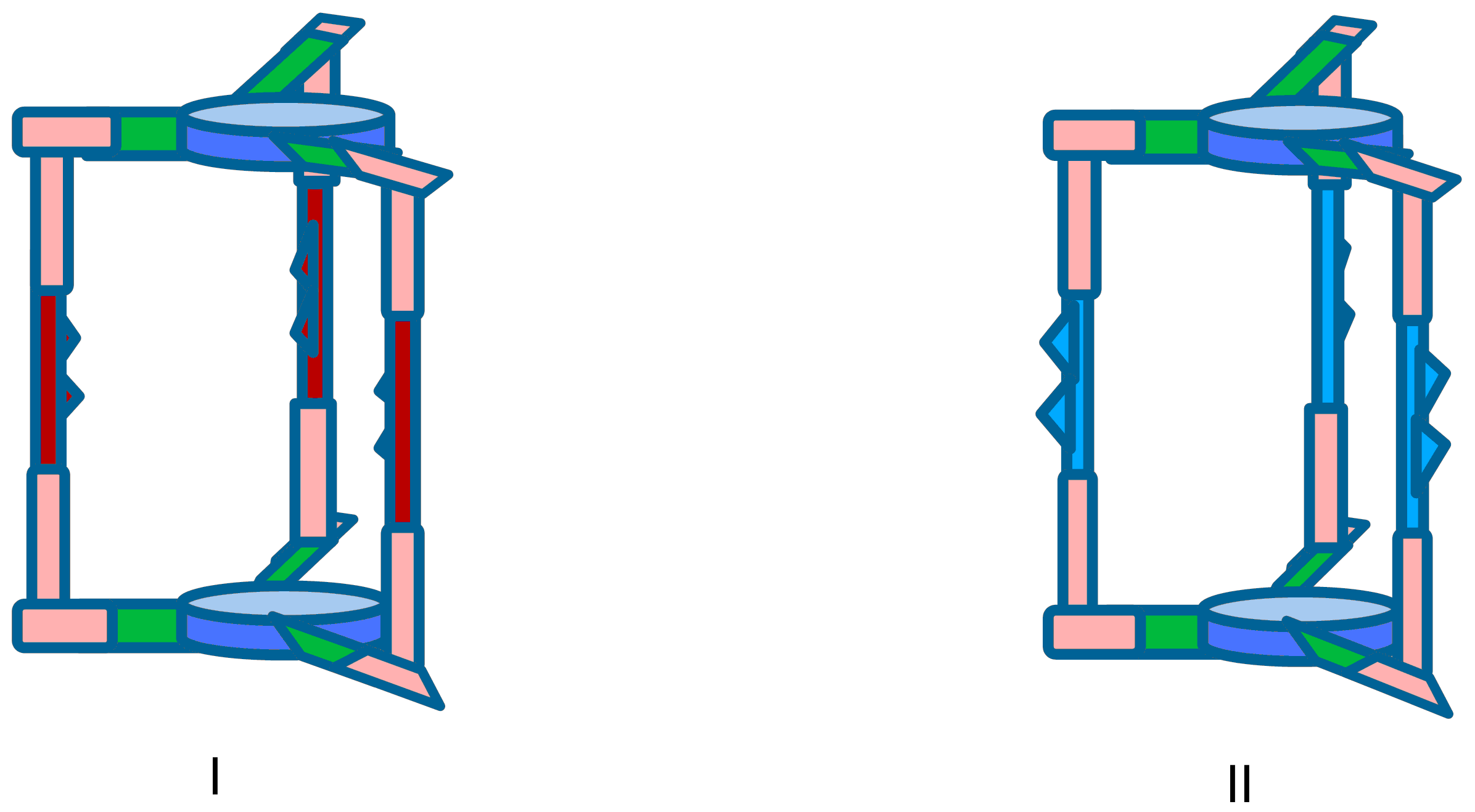
Scheme 3
An aromatic reference group, useful for the obtaining of the cryptands with 4 bridges was also obtained accordingly with scheme 4.
Scheme 3
Woiczechowski-Pop, A.; Gligor, D.; Bende, A.; Varodi, C.; Bogdan, E.; Terec, A.; Grosu, I. Synthesis, Structure, Electrochemical Behavior and Electrochemical Investigations on the Assembling with Pyrene of a Novel C3 Cryptand; Supramol. Chem., 2014, DOI: 10.1080/10610278.2014.904868 .
Crisan, C.; Hadade, D. N.; Terec, A.; Grosu, I., Cryptands with triazine central units and oligoethyleneoxyde bridges: synthesis, structure and complexation abilities, submitted (Tetrahedron Lett.).
Dissemination
Papers
ReportsIn order to determine the reactivity of the target aromatic units, the procedures of macrocyclization and of the requested investigations for the structure determinations some cryptands exhibiting the target aromatic units and having oligoethyleneoxide bridges were obtained in one or two steps accordingly with Scheme 1.

Scheme 1
The structure of the compounds were determined using NMR and MS methods and the complexation ability was investigated using ESI-MS techniques.
For the obtaining of the target interlocked molecules we changed the aromatic reference groups (replacing the triazine unit with a benzene ring) and the bridges (we enlarged the size of the phenantroline derivatives in order to be able to accommodate the target complexes). We decided to use as macrocyclization procedure a metal free click reaction and so we obtained the bridges decorated with azide functions (A and B) and the aromatic reference group was decorated with cyclooctyne moieties (C, Scheme 2).
The click reactions of the azide and cyclooctyne decorated bridges and aromatic reference units lead to the all-in (I) and all-out (II) cryptands in acceptable yields (Scheme 3).
The cryptands were complexed at the phenantroline units with appropriated cations and phenantrolines decorated at positions 2,7 with CH2-N3 groups. The reaction of the complexes with podands having cyclooctyne units at their extremities lead to the desired interlocked molecules. These reactions and products are under investigations.

Scheme 2
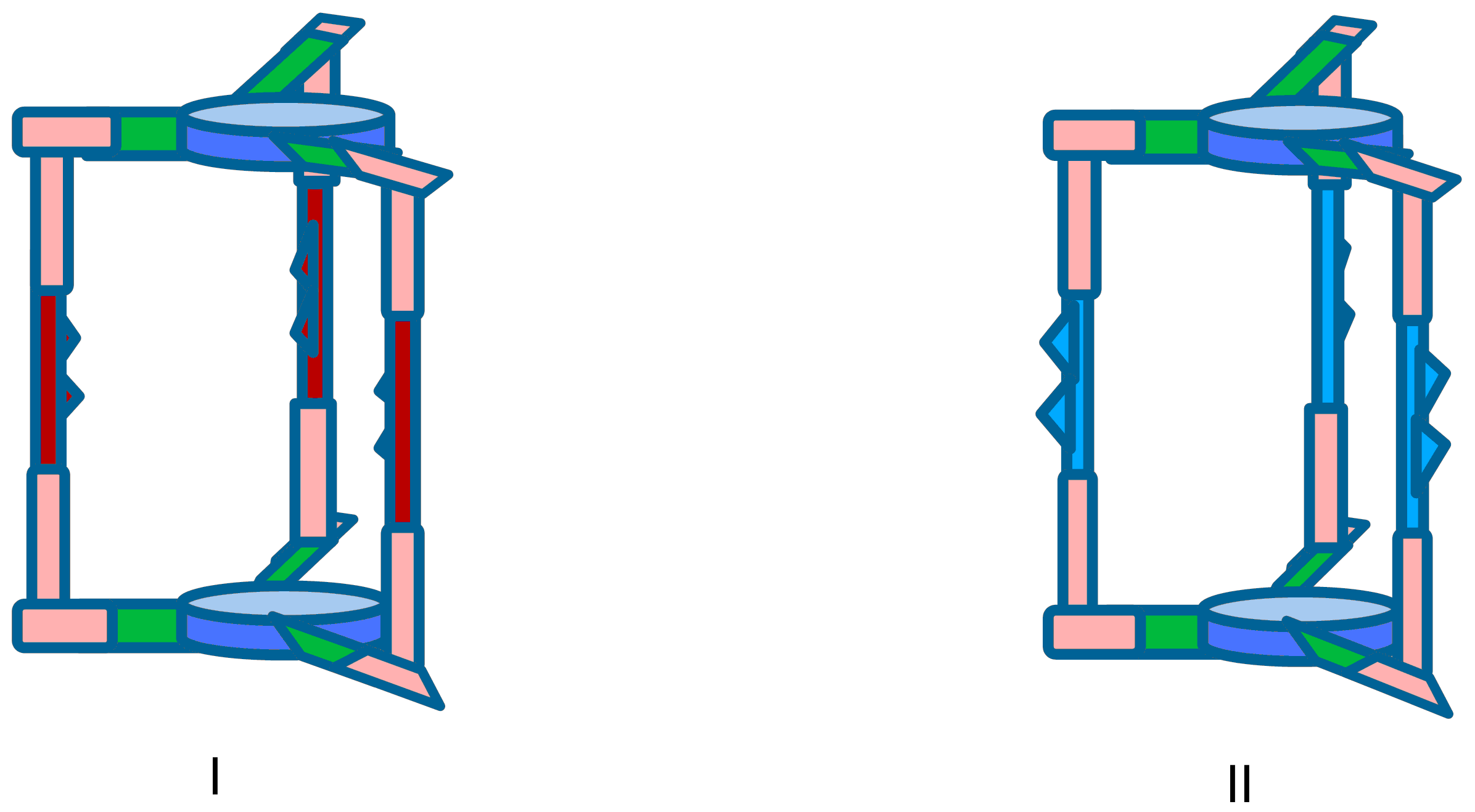
Scheme 3
An aromatic reference group, useful for the obtaining of the cryptands with 4 bridges was also obtained accordingly with scheme 4.

Scheme 3
Woiczechowski-Pop, A.; Gligor, D.; Bende, A.; Varodi, C.; Bogdan, E.; Terec, A.; Grosu, I. Synthesis, Structure, Electrochemical Behavior and Electrochemical Investigations on the Assembling with Pyrene of a Novel C3 Cryptand; Supramol. Chem., 2014, DOI: 10.1080/10610278.2014.904868 .
Crisan, C.; Hadade, D. N.; Terec, A.; Grosu, I., Cryptands with triazine central units and oligoethyleneoxyde bridges: synthesis, structure and complexation abilities, submitted (Tetrahedron Lett.).
Results 2015
A major goal concerning the synthesis of different types of cryptands was connected to the access to aromatic reference units with different functional groups and the large scale obtaining of the reagents for the bridges (the real bottleneck of our targets).
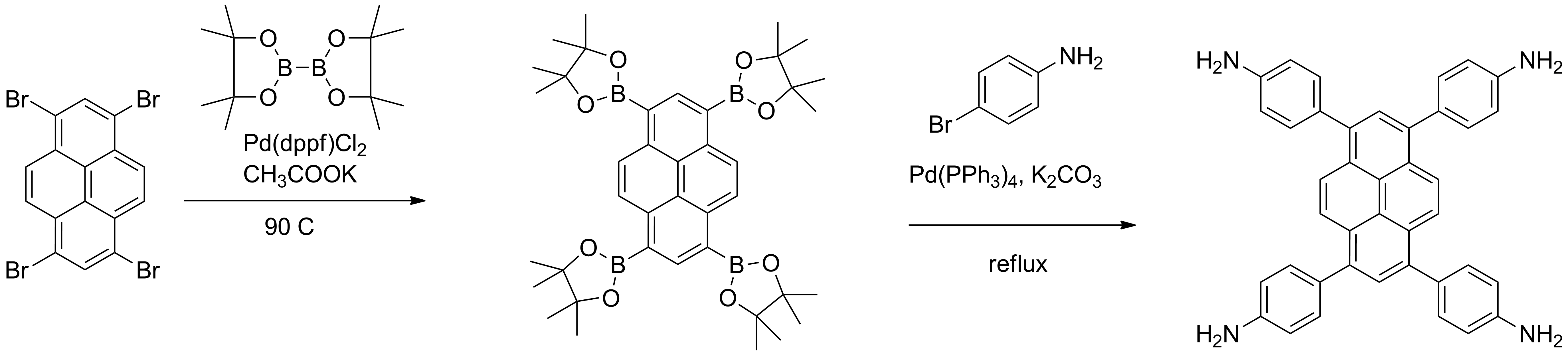
a) Synthesis of tetrasubstituted derivatives of pyrene with one or two types of substituents and of the derivatives of triphenyltriazine bearing two types of substrituents (Scheme 1).
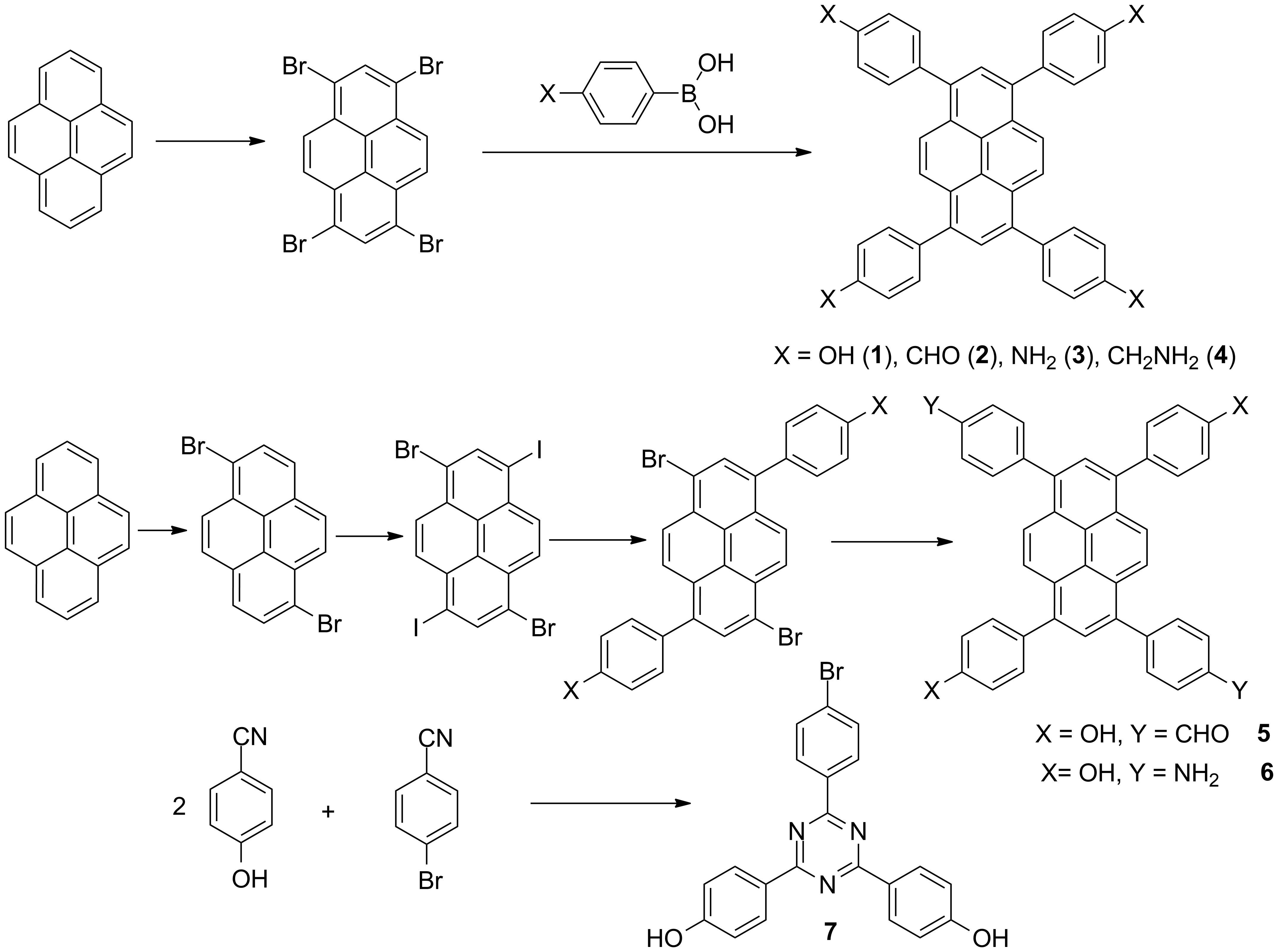
Scheme 1
b) Efficient synthesis of the derivatives of phenantroline designed for in and out bridges (Schemes 2 and 3).
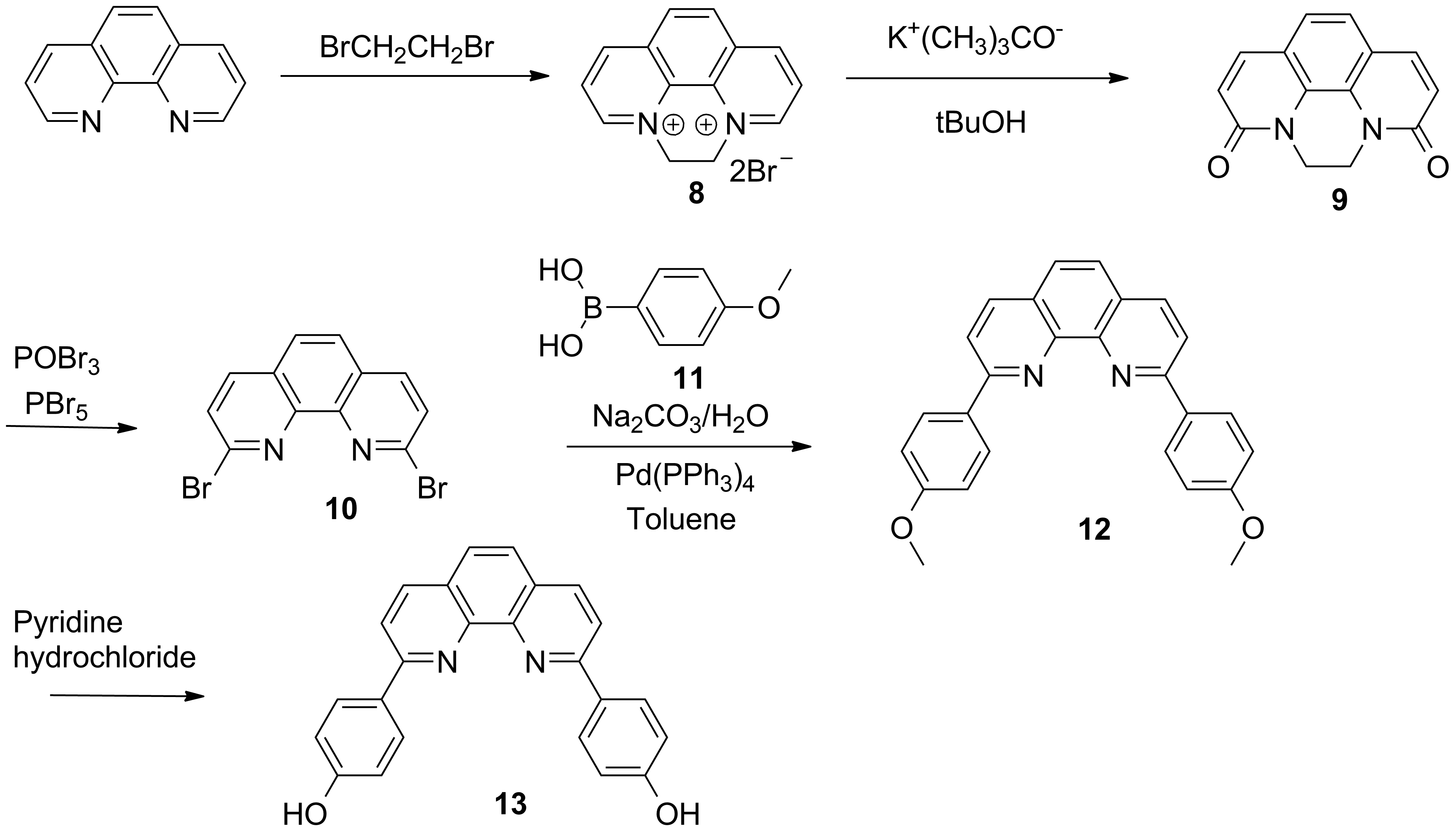
Scheme 2
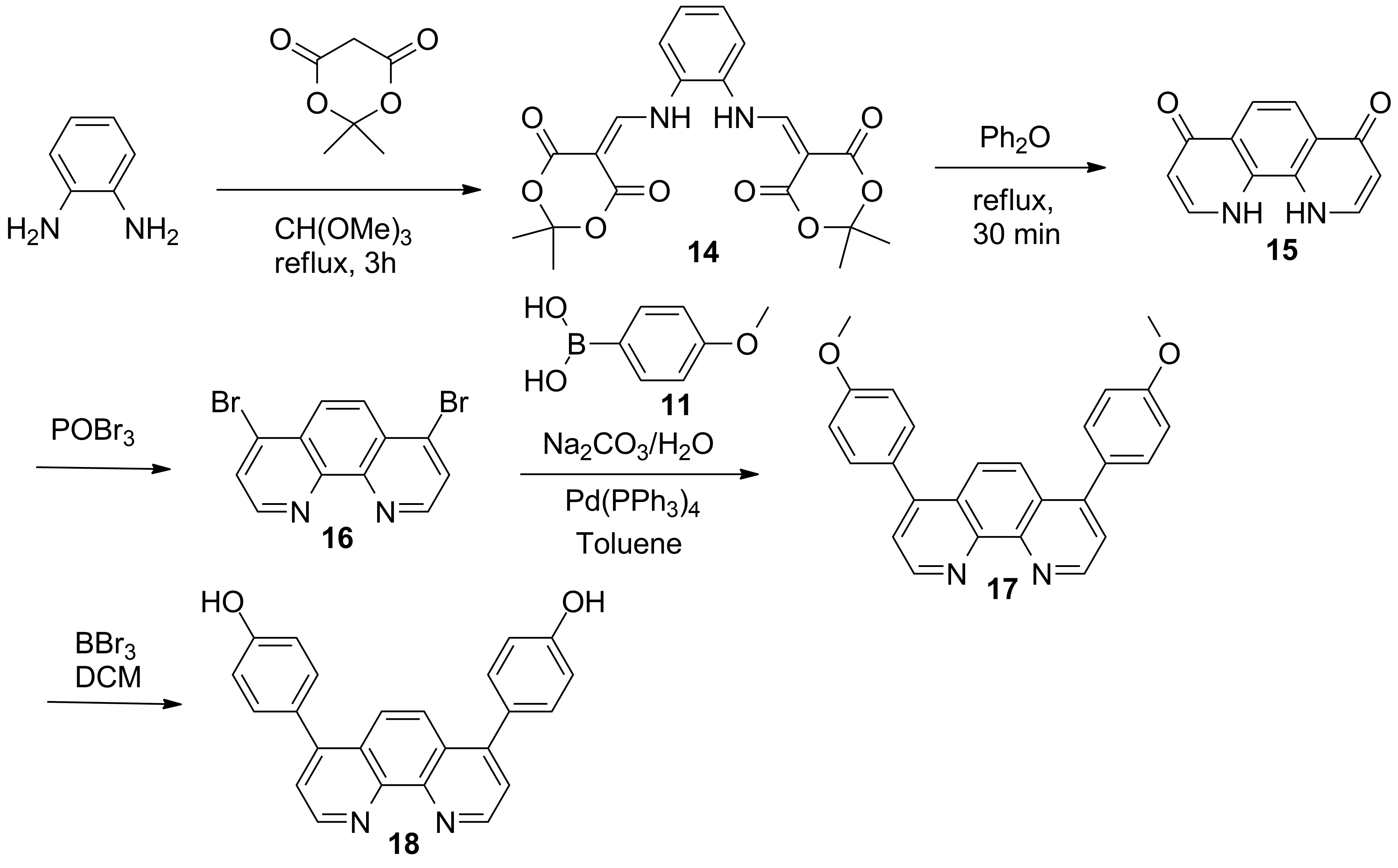
Scheme 3
Another important goal is connected to the development of the active template synthesis for our targets (less steps, simplest reactions).
a) Synthesis of cryptands and linkers for the access to interlocked molecules using the "active-template" approach (Schemes 4-6)[1]
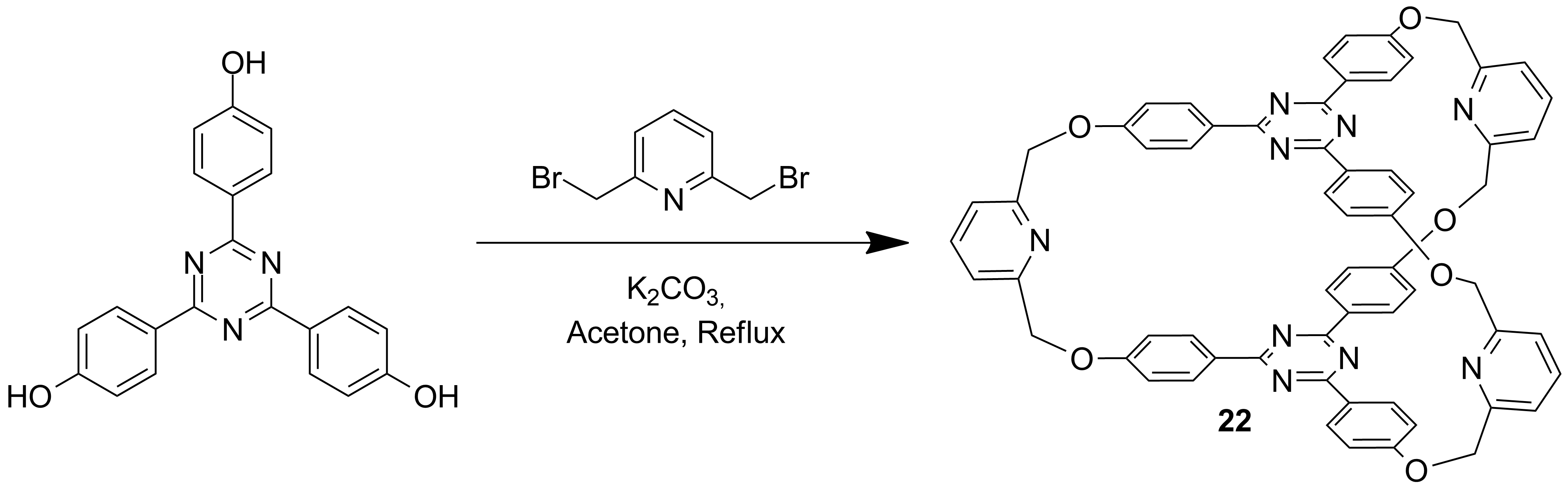
Scheme 4
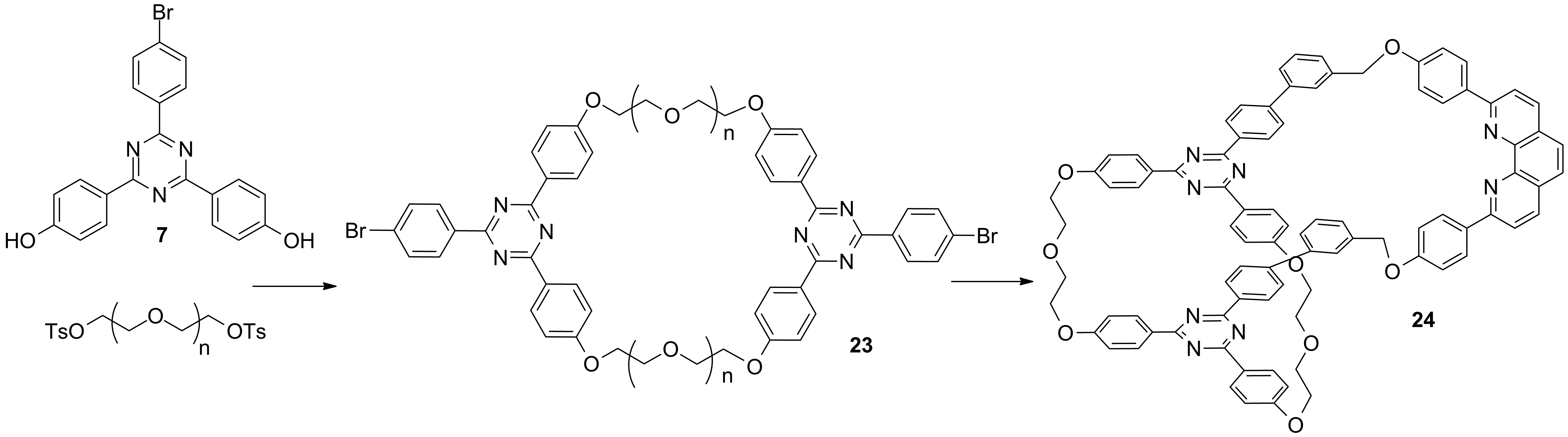
Scheme 5
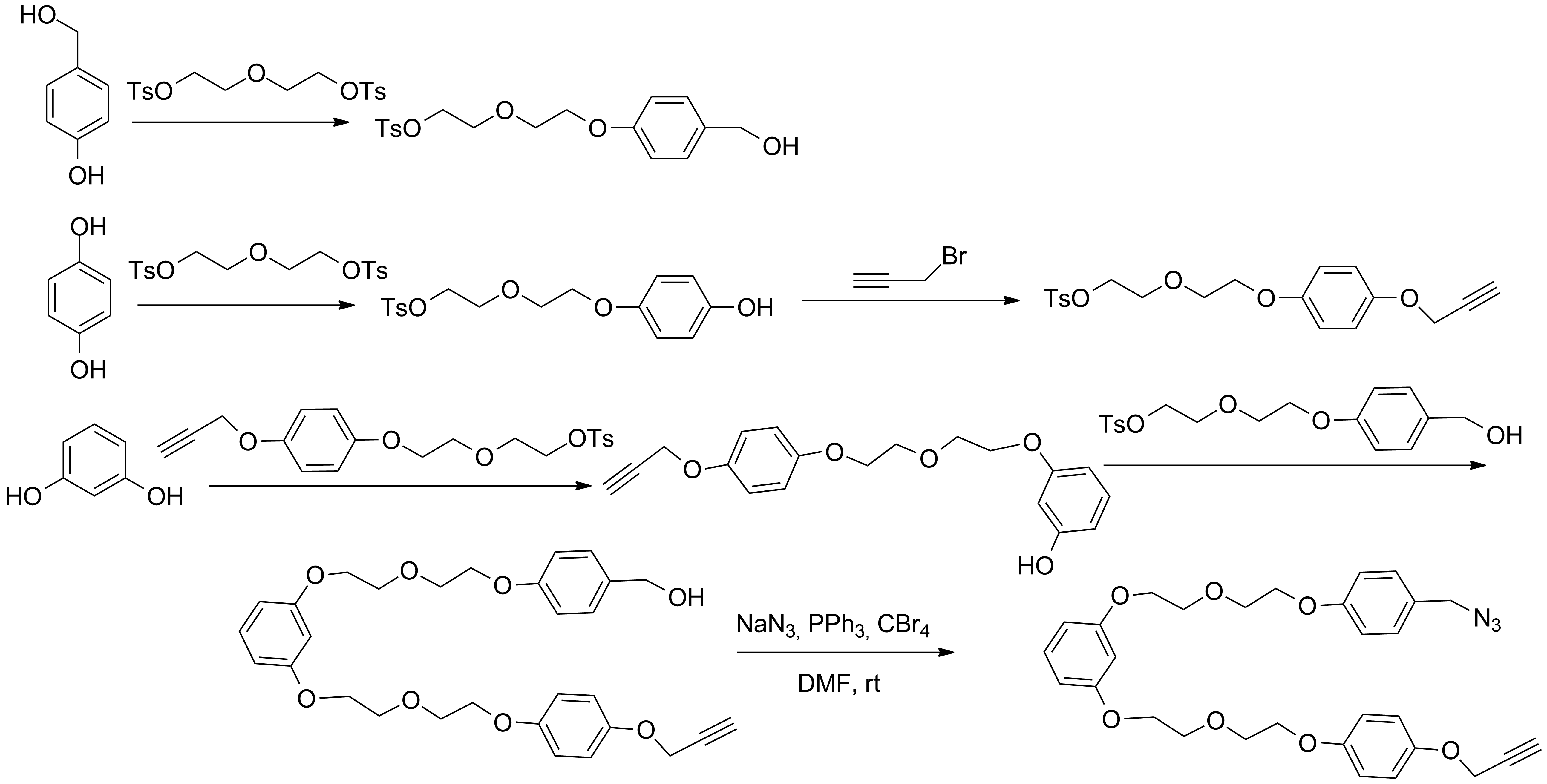
Scheme 6
Other exciting cryptands were obtained in the stage 2014 accordingly to schemes 7 and 8.
In this stage (2015) the complexation ability of these cryptands were investigated using as guests the dications of aromatic diamines. The complexation of the dication of 1,5-nafphthalinediamine was investigated by ES-HRMS and NMR. Figures 1 and 2 show the spectra of the host, guest and host-guest species and the results of the NMR titration (Job-plot) which reveals the 1/1 guest / host ratio.
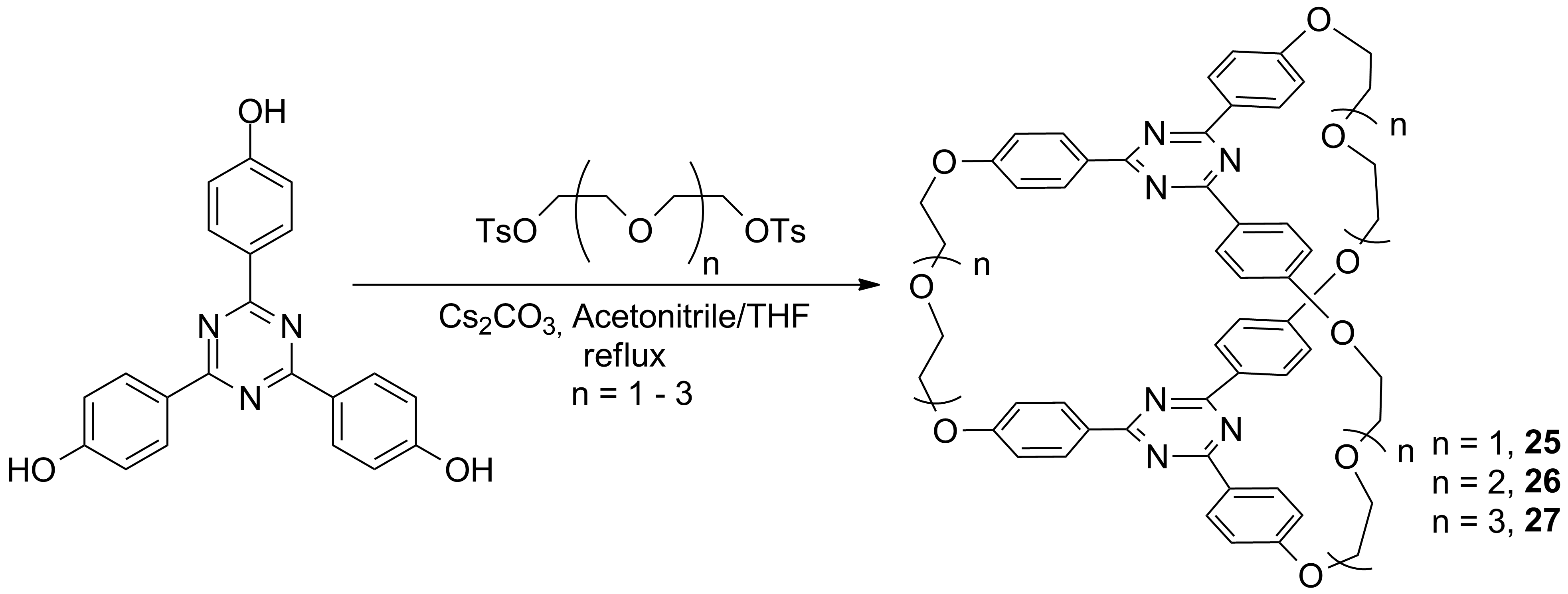
Scheme 7
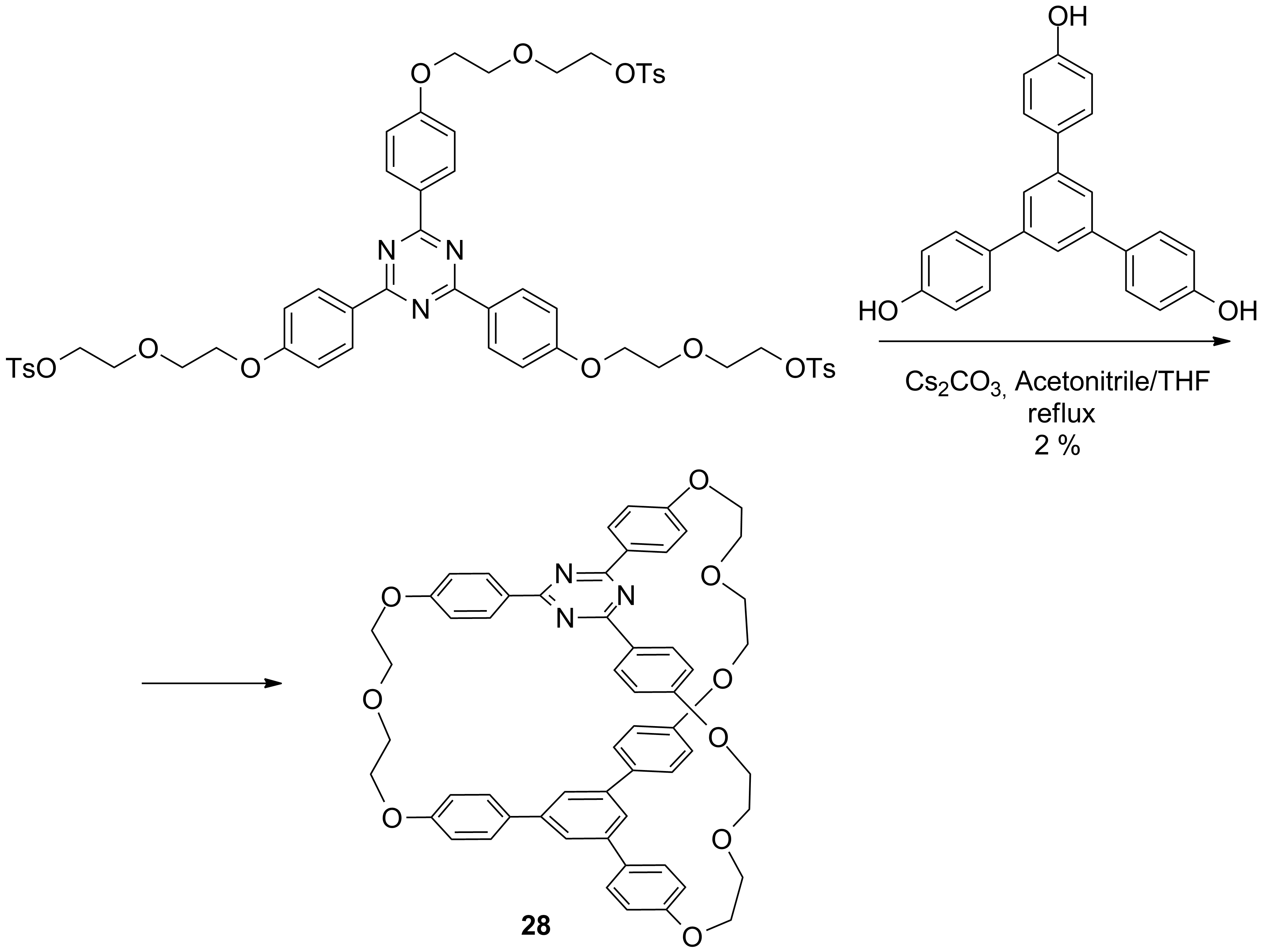
Scheme 8
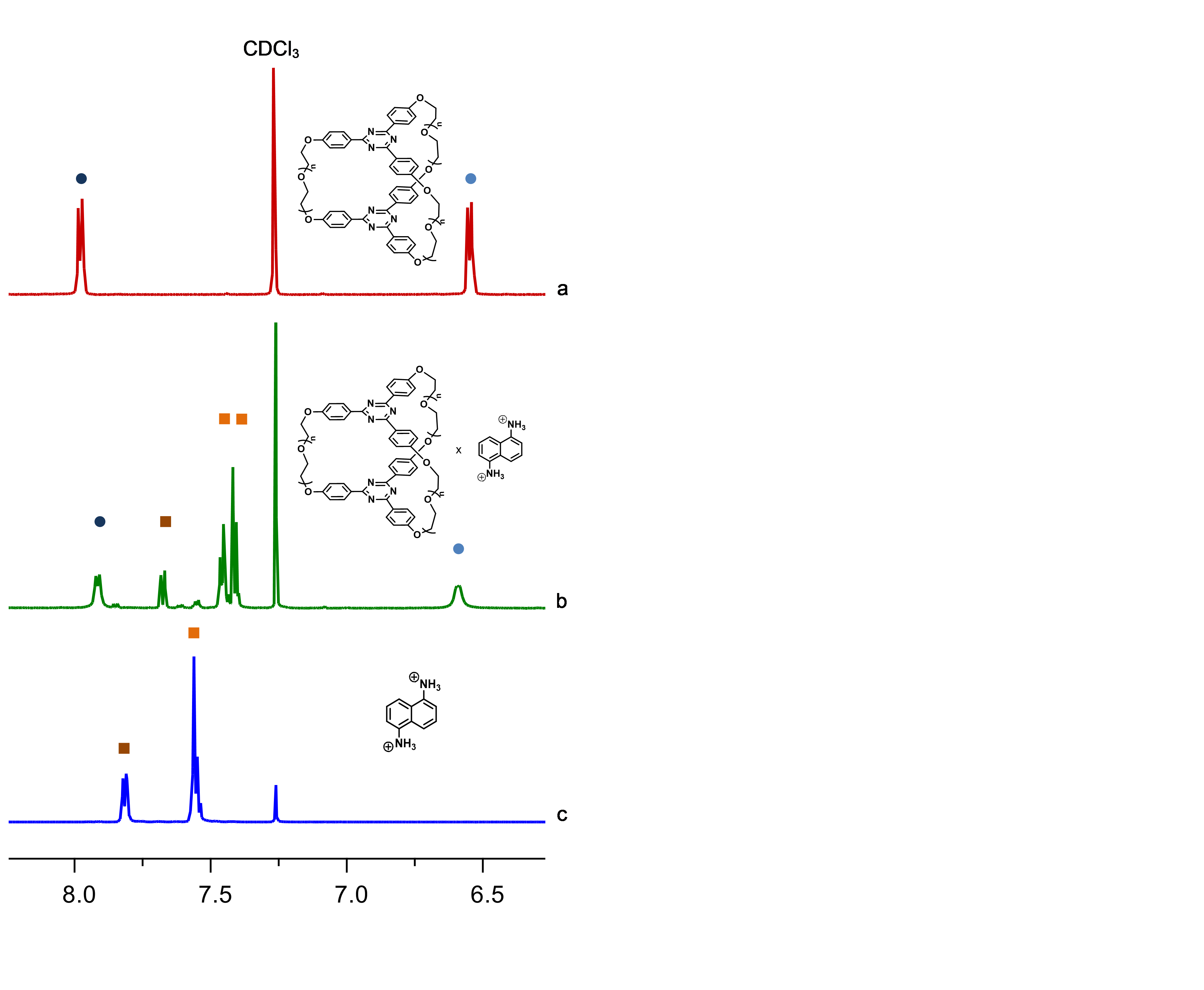
Figure 1. NMR results for the interactions of cryptands 27 with the dication of 1,5-naphthalinediamine.
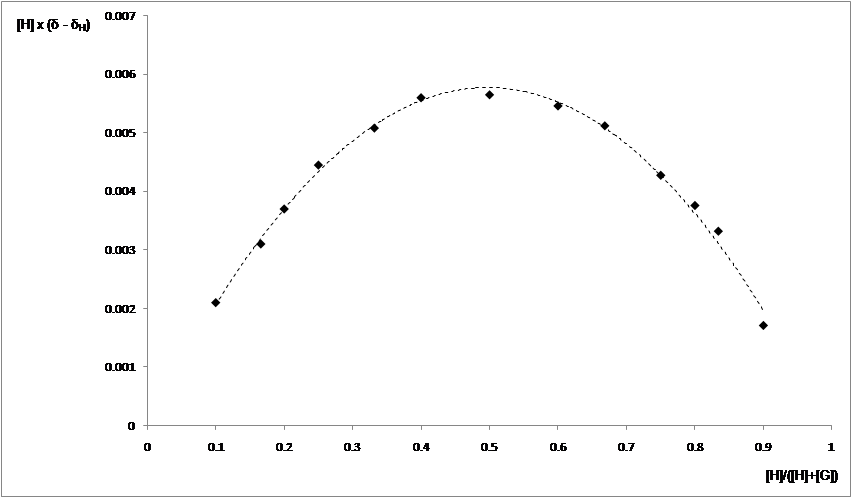
Figure 2. Job plot for 27 (H, 2mM) with the dication NA (G, 2mM) in CD3CN:CDCl3 4:1.
With an “all in” cryptand which was prepared previously we carried out complexation studies using aromatic guests (pyrene, anthracene) in order to explore the methodology for the investigation of the interactions among cryptands and aromatic guests (Scheme 9). The used methods were based on NMR, UV-VIS and Cyclic Voltammetry.
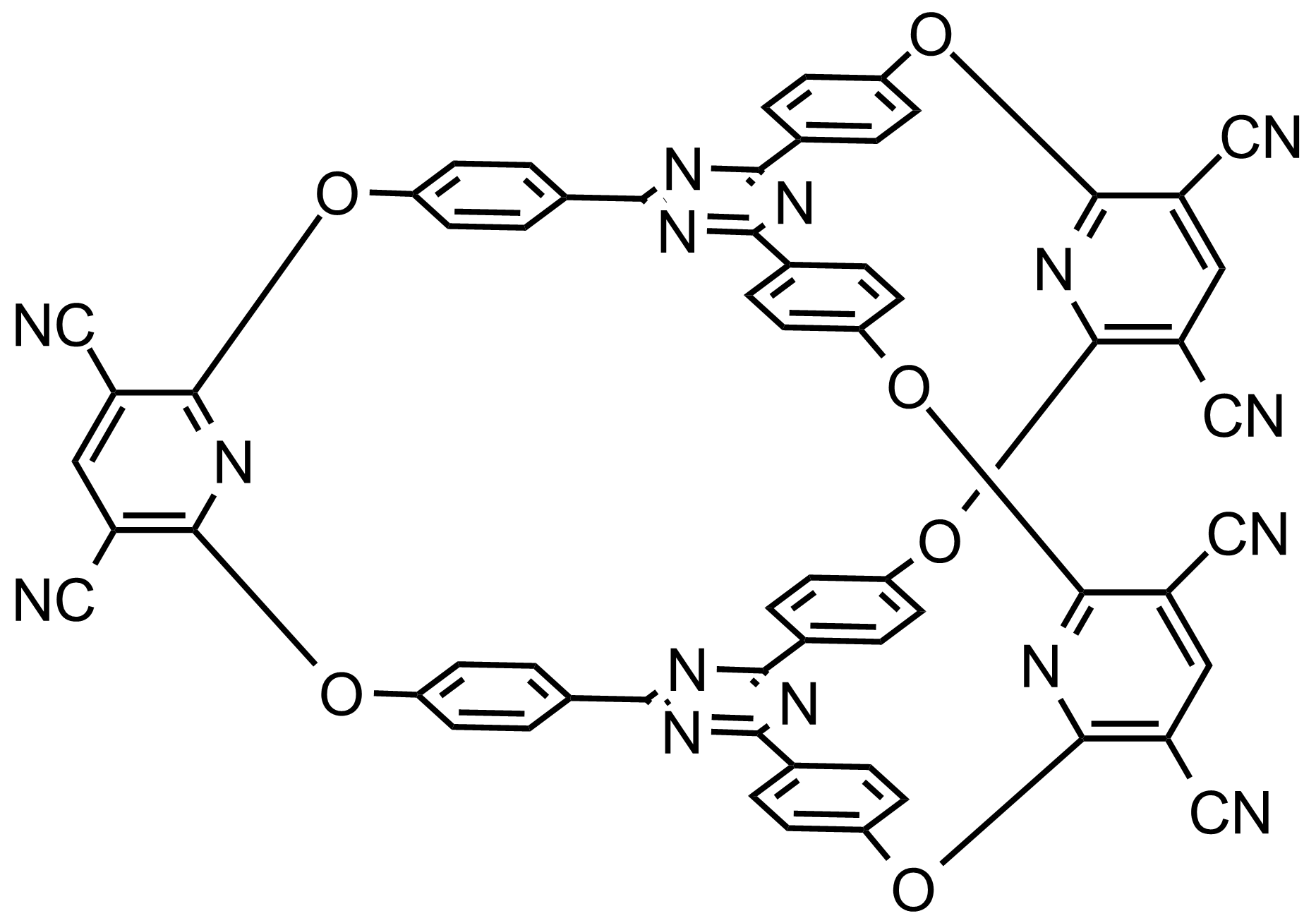
Scheme 9
[1] Gil-Ramirez, G.; Leigh, D. A., Stephens, A. J. Angew. Chem. Int. Ed., 2015, 54, 6110-6150.
A major goal concerning the synthesis of different types of cryptands was connected to the access to aromatic reference units with different functional groups and the large scale obtaining of the reagents for the bridges (the real bottleneck of our targets).
a) Synthesis of tetrasubstituted derivatives of pyrene with one or two types of substituents and of the derivatives of triphenyltriazine bearing two types of substrituents (Scheme 1).

Scheme 1
b) Efficient synthesis of the derivatives of phenantroline designed for in and out bridges (Schemes 2 and 3).

Scheme 2

Scheme 3
Another important goal is connected to the development of the active template synthesis for our targets (less steps, simplest reactions).
a) Synthesis of cryptands and linkers for the access to interlocked molecules using the "active-template" approach (Schemes 4-6)[1]

Scheme 4

Scheme 5

Scheme 6
Other exciting cryptands were obtained in the stage 2014 accordingly to schemes 7 and 8.
In this stage (2015) the complexation ability of these cryptands were investigated using as guests the dications of aromatic diamines. The complexation of the dication of 1,5-nafphthalinediamine was investigated by ES-HRMS and NMR. Figures 1 and 2 show the spectra of the host, guest and host-guest species and the results of the NMR titration (Job-plot) which reveals the 1/1 guest / host ratio.

Scheme 7

Scheme 8

Figure 1. NMR results for the interactions of cryptands 27 with the dication of 1,5-naphthalinediamine.

Figure 2. Job plot for 27 (H, 2mM) with the dication NA (G, 2mM) in CD3CN:CDCl3 4:1.
With an “all in” cryptand which was prepared previously we carried out complexation studies using aromatic guests (pyrene, anthracene) in order to explore the methodology for the investigation of the interactions among cryptands and aromatic guests (Scheme 9). The used methods were based on NMR, UV-VIS and Cyclic Voltammetry.

Scheme 9
[1] Gil-Ramirez, G.; Leigh, D. A., Stephens, A. J. Angew. Chem. Int. Ed., 2015, 54, 6110-6150.
Results 2015
Due to the difficulty encountered in the purification process of the previously synthesized target molecules, a new strategy was envisioned.
The “active-metal-template” strategy (in which the heteroatoms from the arms take part to the formation of the required catalyst of the coupling reactions, leading to mechanically interlocked products [Gil-Ramírez, G.; Leigh, D. A., Stephens, A. J. Angew. Chem. Int. Ed., 2015, 54, 6110-6150]) simplifies the synthetic process, reduces the number of steps and leads to smaller and less complex compounds, facilitating their structural characterization along with a more efficient investigation of properties.
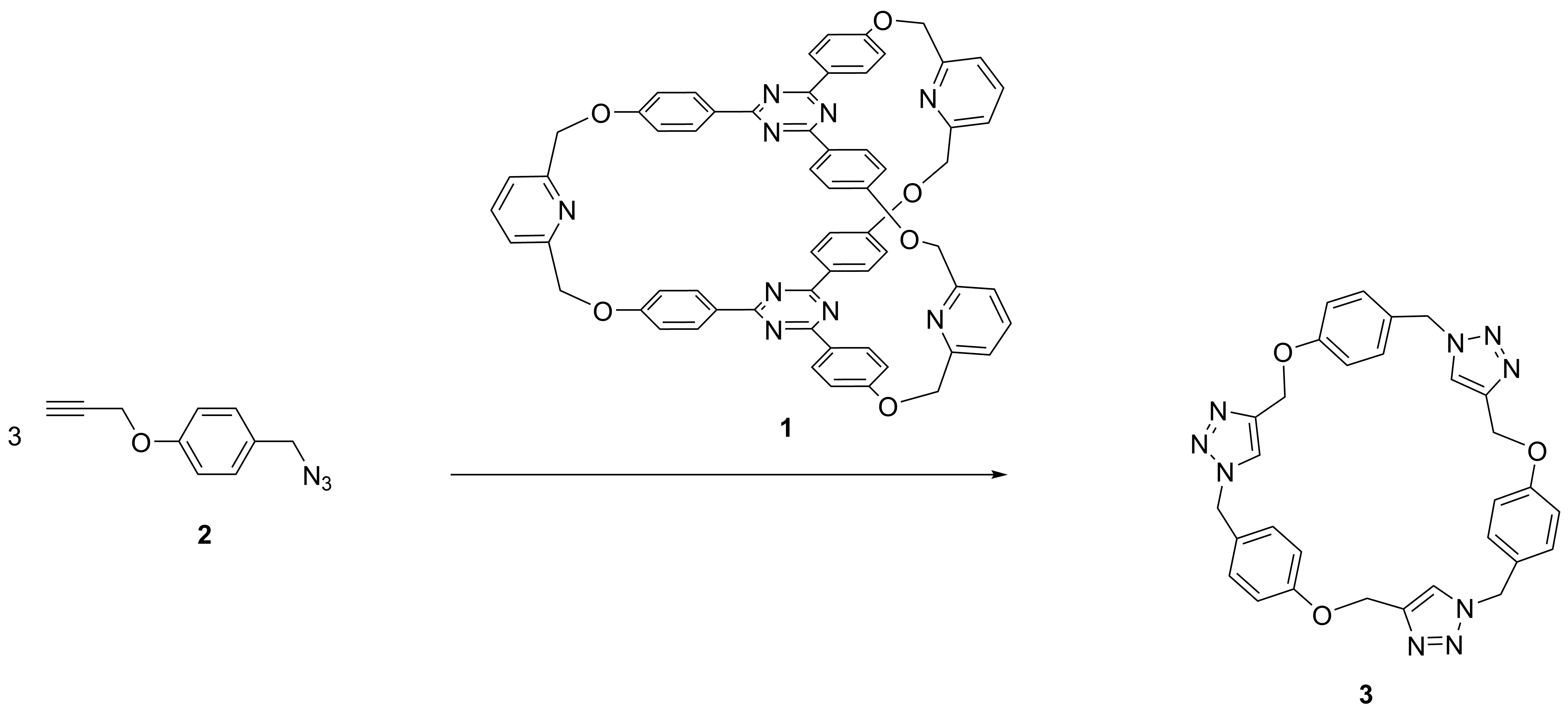
In this context, cryptand 1 and reactant 2 were synthesized in order to test the “active-metal-template” technique and to obtain mechanically interlocked compounds like macrocycles encapsulated inside cryptands (Scheme 1).
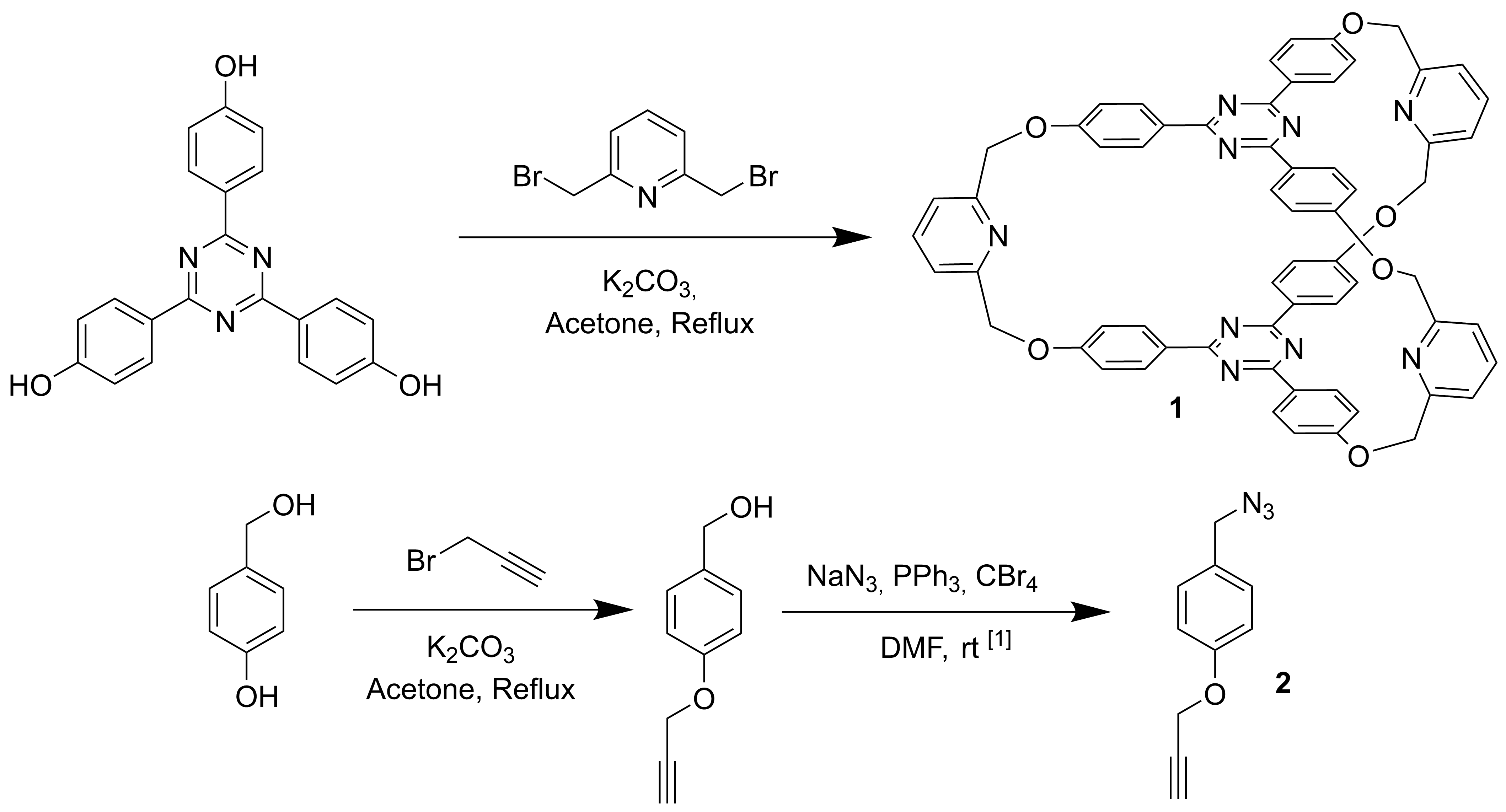
Scheme 1
Having these components, an attempt was made to synthesize a mechanically interlocked derivative, in which the macrocycle would be trapped inside the cavity of the cryptand. The reaction took place with surprisingly high yields (40%) and the trimeric macrocycle 3 (Scheme 2) was exclusively obtained.
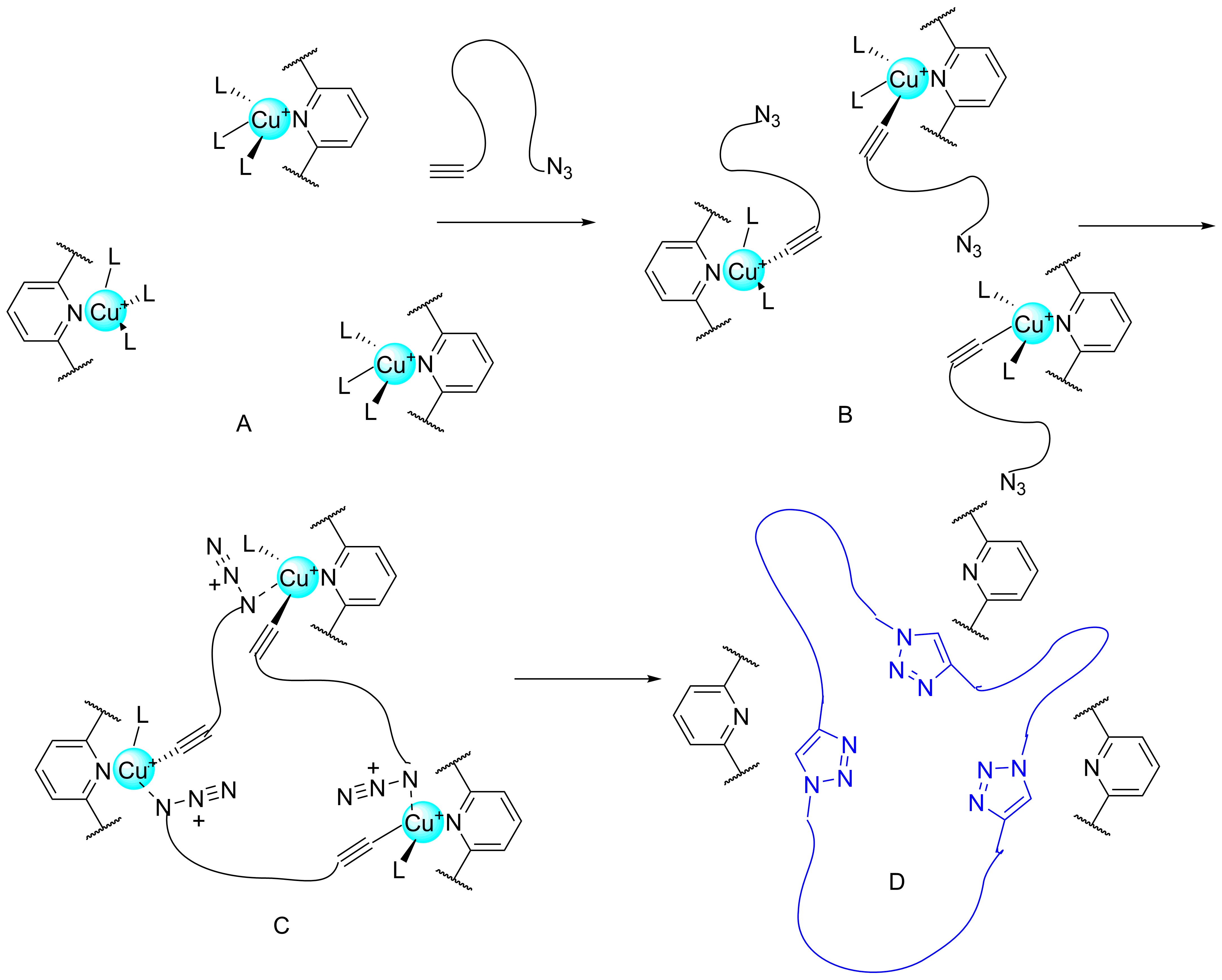
Unfortunately the macrocycle, which undoubtedly was formed (likewise the model from Scheme 3), managed to “escape” the cavity.
Scheme 2
Scheme 3
Job’s plot experiments (1H NMR titrations) performed on compounds 1 and 3 did not show any significant interactions in solution, so, the formation of the mechanically interlocked compound could not be taken into consideration by these experiments. The “click” reaction of 2 in presence of pyridine instead of derivative 1, determined the formation of the macrocycle in lower yields (15%) and the formation of dimers, trimers and tetramers (identified in ESI-MS) as main products was observed. Even the reaction did not yield the desired mechanically interlocked compound, the result is particularly interesting due to its application of “active-metal-template” strategy in obtaining well-defined compounds like the previously mentioned trimer. This aspect has to be further investigated and the results will be published. In order to highlight the result and to obtain new compounds similar to the ones proposed in the project, we synthesized reactants of type 2 with longer arms, such as 4 and 5, (Scheme 4 and 5), capable of reacting either with cryptands of type 1 or with analogue cryptands having a single arm with heterocyclic units (6, n = 2, Scheme 6).
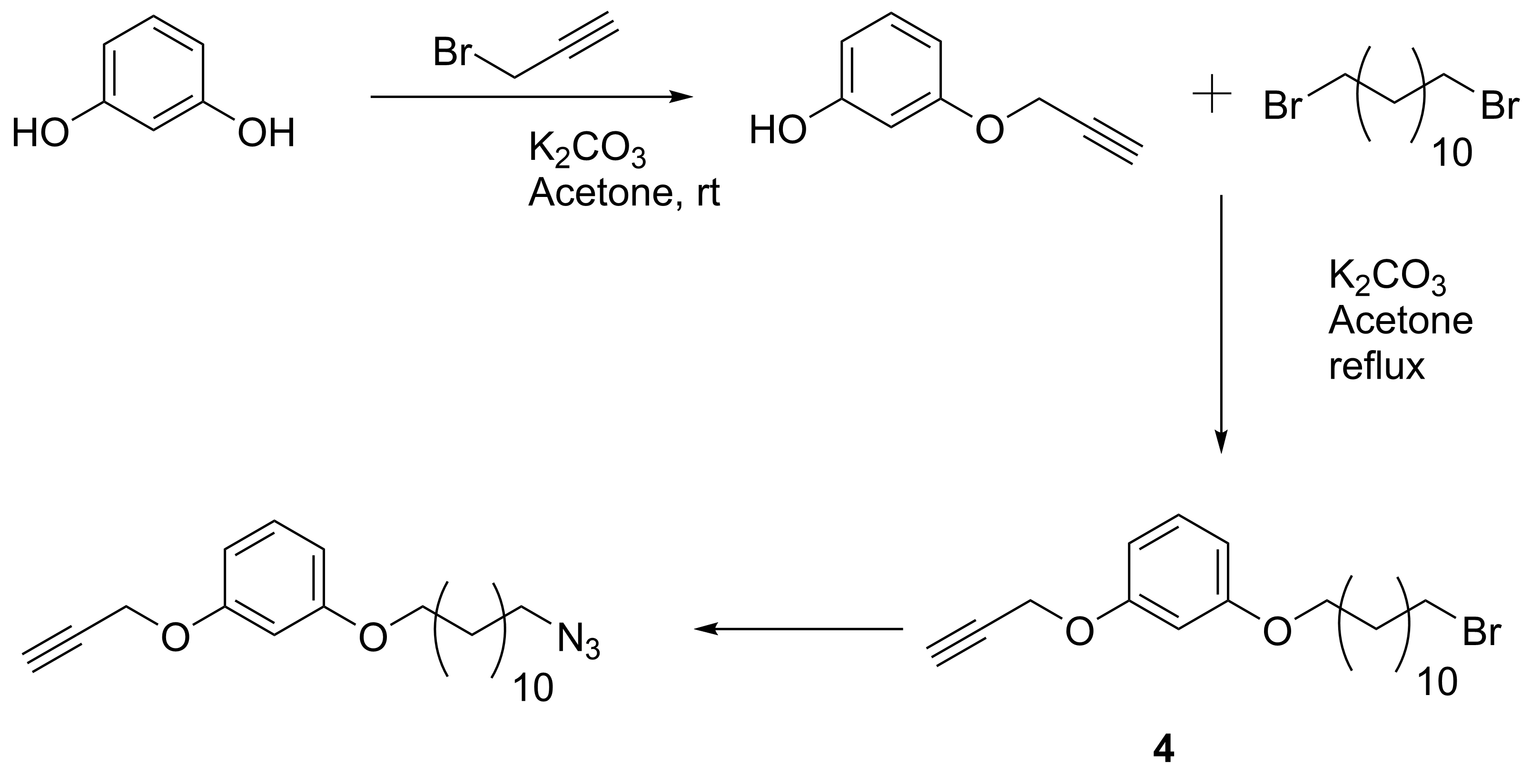
Scheme 4
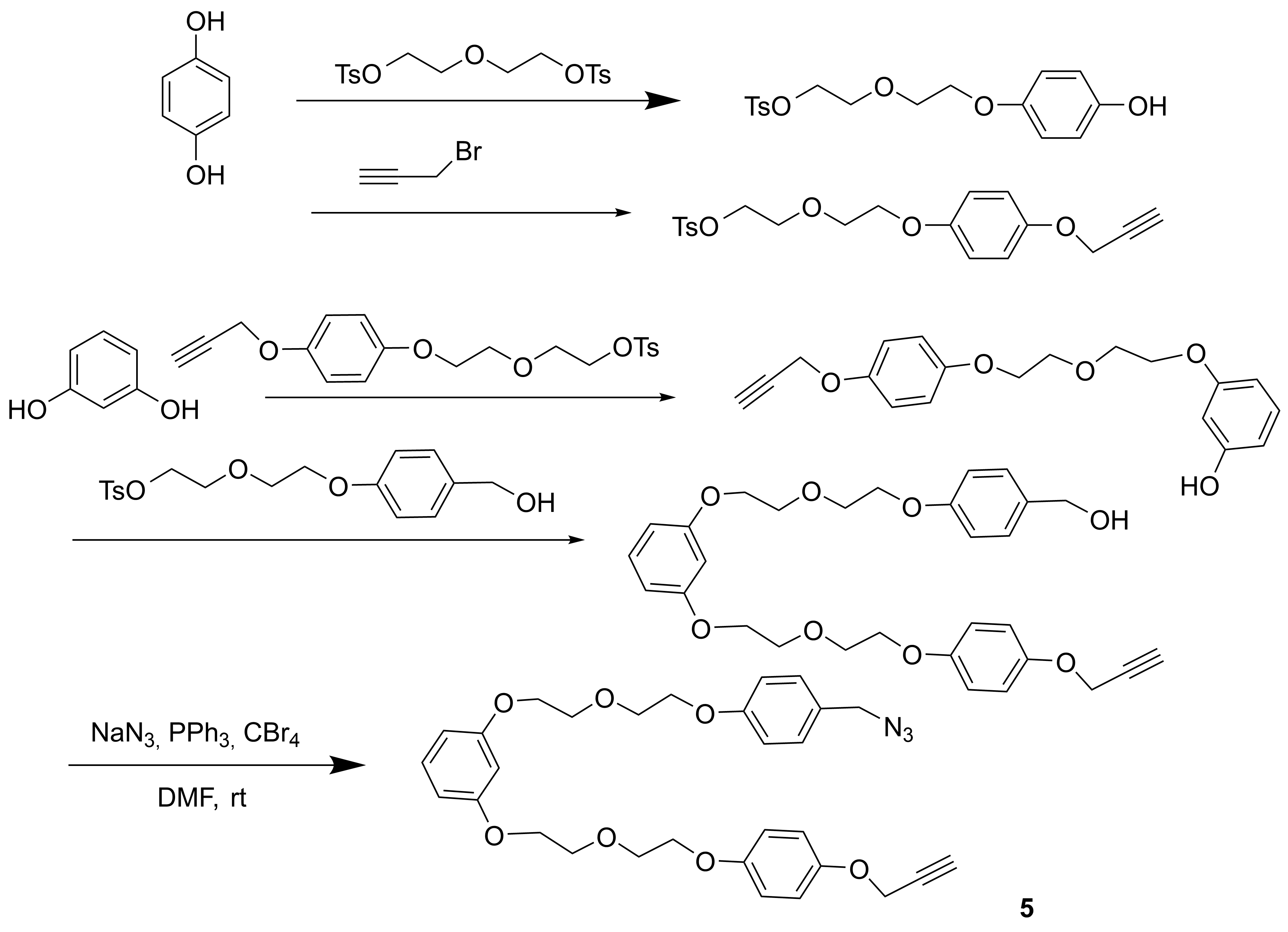
Scheme 5
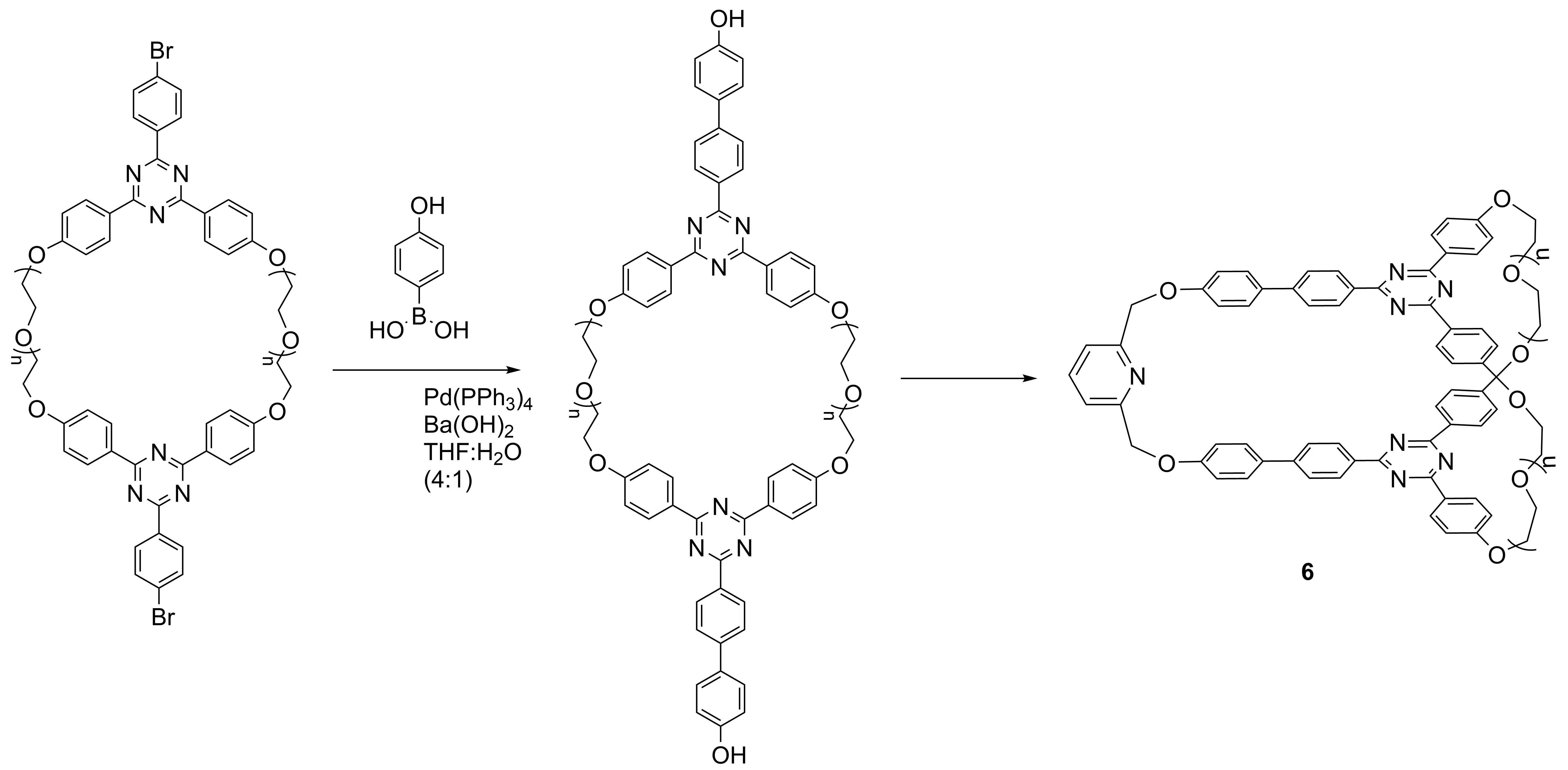
Scheme 6
The macrocycles 1, 6 and compounds 4, 5 will be subjected to the “active-metal template” procedure in order to obtain cryptands in which the macrocycle will be able to perform movement controlled by supramolecular interactions, which involves various units of the macrocycle. The controlling will be made by complexation-decomplexation process or by modifying the pH.
The main objective of strategy D facilitates the obtaining of the desired compounds by using procedures and concepts for more accessible structures as compared to the initially proposed compounds.
Another direction of this research was the synthesis of cyclophanes and cryptands with 1,3-phenothiazine units in arms (Schemes 7 and 8). These compounds contain inwards directed functionalized arms and a priori could lead to the target mechanically interlocked structures. The reaction yields for obtaining cyclophanes 13-16 were good in both used methods, the procedure involving intermediates as well as in the “one pot” procedure.
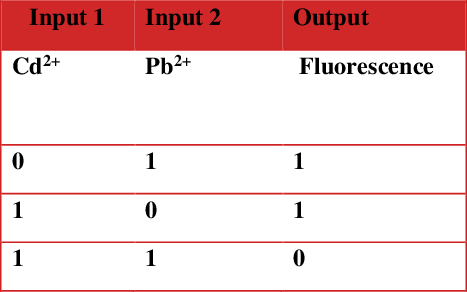
However, in case of cryptand 17 only “one pot” method gave good results. Complexation studies revealed interesting results for cations and organic compounds as guests. Experiments with lead and cadmium cations (Figure 1) have allowed the construction of fluorescent molecular logic gates of type XOR (Table 1)
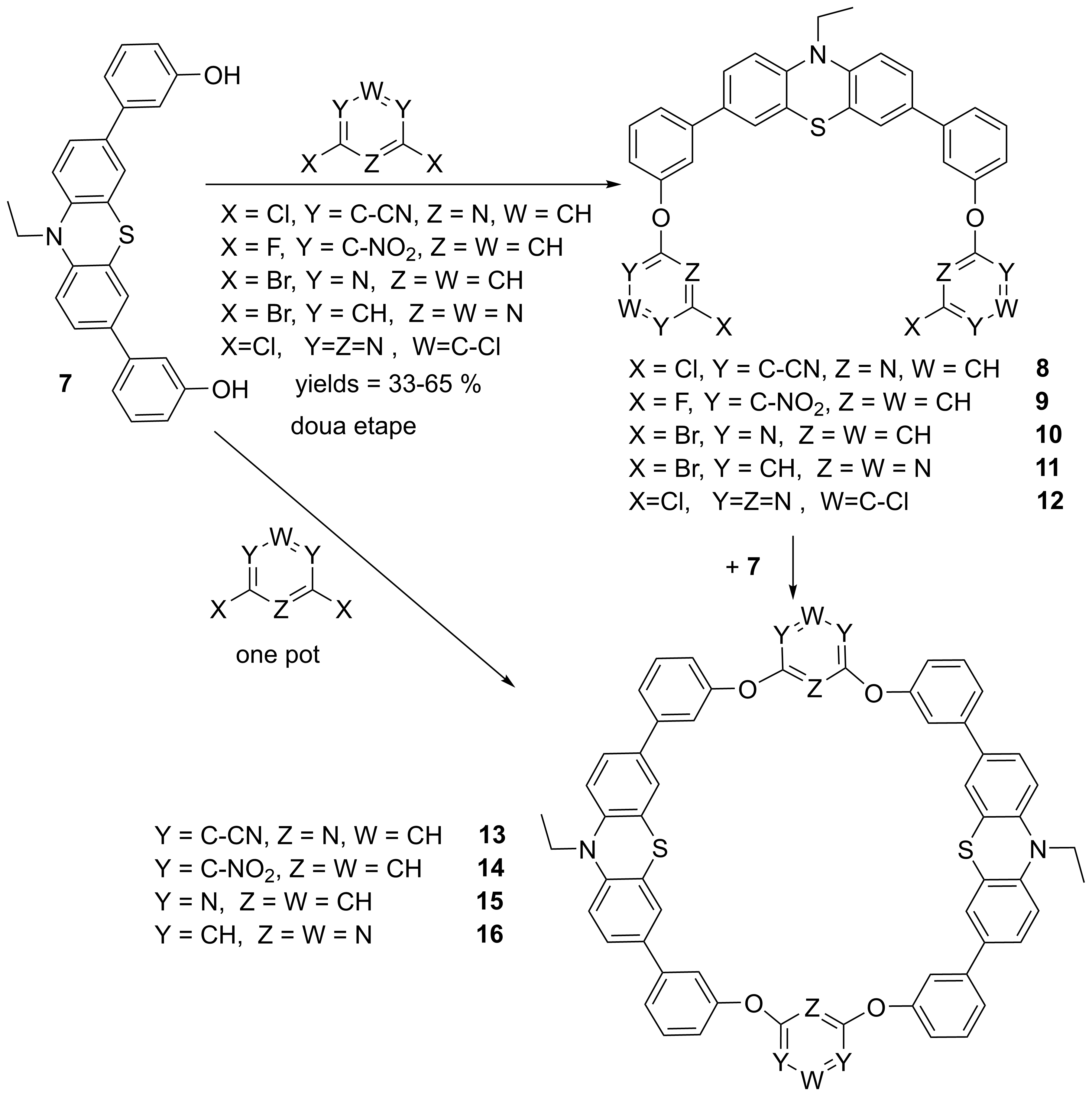
Scheme 7
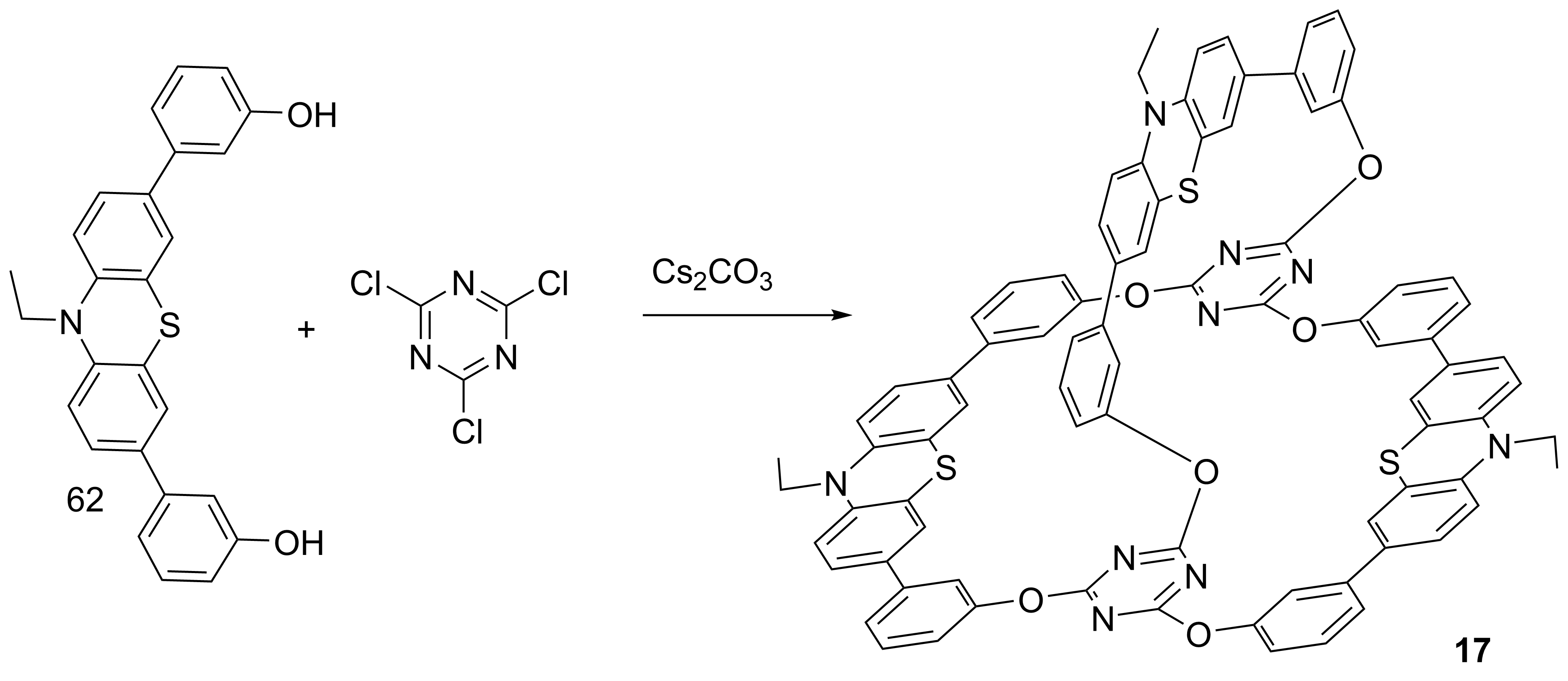
Scheme 8
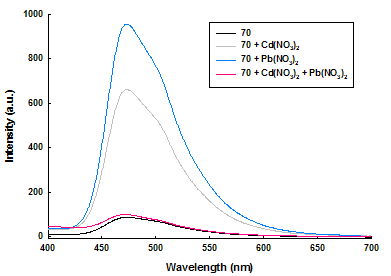
Figure 1. Emission spectra of cyclophane 15 in presence of Cd2+, Pb2+.
Phenothiazine-based cyclophanes and cryptands showed high versatility for performing synthesis of mechanically interlocked molecules. These experiments will be further continued.
In order to achieve project objectives other substrates were taken into consideration and several Bannister type macrocycles (Scheme 9) having ortho-disubstituted biphenylic units were obtained via oxime derivative. We expected the reactions will lead to dimers (2+2 reaction), which would have been interesting compounds for project. Surprisingly, only monomers resulted from the reaction. These compounds were investigated by NMR spectroscopy, MS spectrometry and the enantiomers were discriminated on chiral columns. The compounds exhibit affinity for various cations, and this property has been demonstrated both by NMR titration and by fluorescence investigations. Also for this case, the “active template” experiments will be performed.
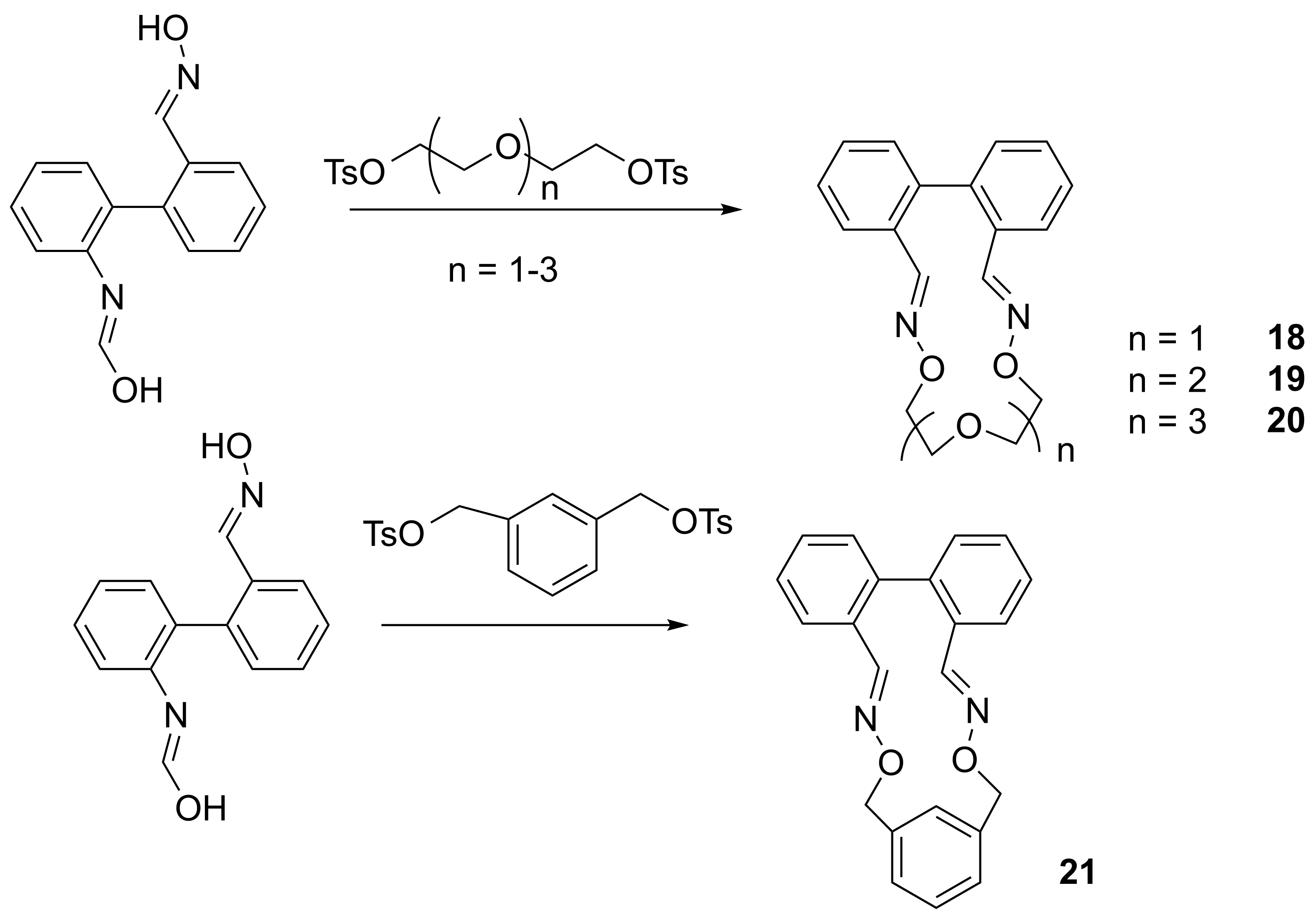
Scheme 9
In conclusion, the project objectives have been achieved and the proposed activities were carried out such that some of the target compounds were obtained and investigated, while the rest of investigations have to be further completed. The “activ-template” technique was proved to be the most effective and the results obtained by this method are notable and will be next published.
So far, 3 articles were published in organic chemistry and supramolecular chemistry related journals and other 3 articles will be sent in order to be published this year (the investigations are in the final stage at this moment).
At least two other papers will be published with the results of the undergoing experiments. In project were involved three PhD students and more Master‘s and Bachelor students. The dissemination activity has been also achieved by five plenary conferences, keynote or invited professors and several oral presentations and posters.
Due to the difficulty encountered in the purification process of the previously synthesized target molecules, a new strategy was envisioned.
The “active-metal-template” strategy (in which the heteroatoms from the arms take part to the formation of the required catalyst of the coupling reactions, leading to mechanically interlocked products [Gil-Ramírez, G.; Leigh, D. A., Stephens, A. J. Angew. Chem. Int. Ed., 2015, 54, 6110-6150]) simplifies the synthetic process, reduces the number of steps and leads to smaller and less complex compounds, facilitating their structural characterization along with a more efficient investigation of properties.
In this context, cryptand 1 and reactant 2 were synthesized in order to test the “active-metal-template” technique and to obtain mechanically interlocked compounds like macrocycles encapsulated inside cryptands (Scheme 1).

Scheme 1
Having these components, an attempt was made to synthesize a mechanically interlocked derivative, in which the macrocycle would be trapped inside the cavity of the cryptand. The reaction took place with surprisingly high yields (40%) and the trimeric macrocycle 3 (Scheme 2) was exclusively obtained.
Unfortunately the macrocycle, which undoubtedly was formed (likewise the model from Scheme 3), managed to “escape” the cavity.

Scheme 2

Scheme 3
Job’s plot experiments (1H NMR titrations) performed on compounds 1 and 3 did not show any significant interactions in solution, so, the formation of the mechanically interlocked compound could not be taken into consideration by these experiments. The “click” reaction of 2 in presence of pyridine instead of derivative 1, determined the formation of the macrocycle in lower yields (15%) and the formation of dimers, trimers and tetramers (identified in ESI-MS) as main products was observed. Even the reaction did not yield the desired mechanically interlocked compound, the result is particularly interesting due to its application of “active-metal-template” strategy in obtaining well-defined compounds like the previously mentioned trimer. This aspect has to be further investigated and the results will be published. In order to highlight the result and to obtain new compounds similar to the ones proposed in the project, we synthesized reactants of type 2 with longer arms, such as 4 and 5, (Scheme 4 and 5), capable of reacting either with cryptands of type 1 or with analogue cryptands having a single arm with heterocyclic units (6, n = 2, Scheme 6).

Scheme 4

Scheme 5

Scheme 6
The macrocycles 1, 6 and compounds 4, 5 will be subjected to the “active-metal template” procedure in order to obtain cryptands in which the macrocycle will be able to perform movement controlled by supramolecular interactions, which involves various units of the macrocycle. The controlling will be made by complexation-decomplexation process or by modifying the pH.
The main objective of strategy D facilitates the obtaining of the desired compounds by using procedures and concepts for more accessible structures as compared to the initially proposed compounds.
Another direction of this research was the synthesis of cyclophanes and cryptands with 1,3-phenothiazine units in arms (Schemes 7 and 8). These compounds contain inwards directed functionalized arms and a priori could lead to the target mechanically interlocked structures. The reaction yields for obtaining cyclophanes 13-16 were good in both used methods, the procedure involving intermediates as well as in the “one pot” procedure.
However, in case of cryptand 17 only “one pot” method gave good results. Complexation studies revealed interesting results for cations and organic compounds as guests. Experiments with lead and cadmium cations (Figure 1) have allowed the construction of fluorescent molecular logic gates of type XOR (Table 1)

Scheme 7

Scheme 8

Figure 1. Emission spectra of cyclophane 15 in presence of Cd2+, Pb2+.
Table 1. Table of true for the potential XOR logic gate of 15.

Phenothiazine-based cyclophanes and cryptands showed high versatility for performing synthesis of mechanically interlocked molecules. These experiments will be further continued.
In order to achieve project objectives other substrates were taken into consideration and several Bannister type macrocycles (Scheme 9) having ortho-disubstituted biphenylic units were obtained via oxime derivative. We expected the reactions will lead to dimers (2+2 reaction), which would have been interesting compounds for project. Surprisingly, only monomers resulted from the reaction. These compounds were investigated by NMR spectroscopy, MS spectrometry and the enantiomers were discriminated on chiral columns. The compounds exhibit affinity for various cations, and this property has been demonstrated both by NMR titration and by fluorescence investigations. Also for this case, the “active template” experiments will be performed.

Scheme 9
In conclusion, the project objectives have been achieved and the proposed activities were carried out such that some of the target compounds were obtained and investigated, while the rest of investigations have to be further completed. The “activ-template” technique was proved to be the most effective and the results obtained by this method are notable and will be next published.
So far, 3 articles were published in organic chemistry and supramolecular chemistry related journals and other 3 articles will be sent in order to be published this year (the investigations are in the final stage at this moment).
At least two other papers will be published with the results of the undergoing experiments. In project were involved three PhD students and more Master‘s and Bachelor students. The dissemination activity has been also achieved by five plenary conferences, keynote or invited professors and several oral presentations and posters.
Dissemination
Papers
-
Woiczechowski-Pop, A.; Gligor, D.; Bende, A.; Varodi, C.; Bogdan, E.; Terec, A.; Grosu, I. Synthesis, Structure,Electrochemical Behavior and Electrochemical Investigations on the Assembling with Pyrene of a Novel C3 Cryptand; Supramol. Chem., 2014, DOI: 10.1080/10610278.2014.904868
-
Crisan, C.V., Terec, A., Hadade, N.D., Grosu, I., Cryptands with 2,4,6-tris(p-phenylene)-1,3,5-triazine central units and oligoethyleneoxide bridges: Synthesis, structure and complexation abilities; Tetrahedron, 2015, 71, 6888-6893.
-
Woiczechowski-Pop, A., Gligor, D., Bende, A., Varodi, C., Bogdan, E., Terec, A., Grosu, I., Synthesis, structure, electrochemical behaviour and electrochemical investigations on the assembling with pyrene of a novel C3 cryptand, Supramol. Chem., 2015, 27, 52-58.
-
Crisan, C.V., Terec, A., Hadade, N.D., Grosu, I. Cryptands with 2,4,6-tris(p-phenylene)-1,3,5-triazine central units and oligoethyleneoxide bridges: Synthesis, structure and complexation abilities, Tetrahedron, 2015, 71, 6888-6893.
-
Bogdan, E.; Hadade, N. D.; Terec, A.; Grosu I., The 1,3-Dioxane Motif - a Useful Tool in Monitoring Molecular and Supramolecular Architectures, Tetrahedron Lett., 2016, 57, 2683-2691.
-
Woiczechowski-Pop, A., Lar, C., Bende, A., Grosu, I.-G., Bogdan, E., Terec, A., Hadade, N.D., Grosu, I., Synthesis, structure and host-guest supramolecular assemblies with aromatic guests of a new cryptand exhibiting triazine and pyridine aromatic rings, Supramol. Chem., to be submitted in 2016.
-
Rednic, M. I., Hadade, N. D., Terec, A., Diac, A. P., Grosu, I. XOR logical gates based on novel cyclophanes and cryptands with phenothiazine units in the bridges Org. Biomol. Chem., to be submitted in 2016.
-
Lar, C.; Bogdan, E.; Terec, A.; Hadade, N. D.; Grosu, I.; David, L.; Paizs, C.; Grosu, I. G. Macrocycles embedding biphenyl units: synthesis, stereochemistry and binding ability Eur. J. Org. Chem., to be submitted in 2016.
-
Prof. dr. Ion Grosu-plenary lecture: Macrocyclic Hosts and H-bonding Self-assembled Aggregates: Synthesis, Structure and Dynamics; A XXXIII-a Conferinta Nationala de Chimie, Caciulata, October 1st-3rd 2014.
-
Prof. dr. Ion Grosu-invited lecture at Technical University of Braunschweig: Cyclophanes, Cryptands and H-bonding Self-assembled Aggregates: Synthesis, Structure and Molecular Machines; June 25th 2014.
-
Prof. dr. Ion Grosu -keynote lecture, 8-ème Colloque Franco - Roumain de Chimie Appliquée, September 15th - 18th 2014, ENSCM, Montpellier, France.
Macrocycles, Cyclophanes, Cryptandes et Architectures Supramoléculaires Obtenues par Liaisons d'Hydrogène.
-
Cosmin V. Crisan, Niculina D. Hadade, Ion Grosu: poster; 1,3,5-Triazine Based Cyclophane Cryptands as Potential Host Molecules, BOSS XIV - 14th Belgian Organic Synthesis Symposium, 13th-18th, 2014, Louvain-la-Neuve, Belgium.
-
Eszter Lakatos, Anamaria Terec, Niculina D. Hadade and Ion Grosu: poster, Synthesis and Characterization of New Building Blocks en Route to Supramolecular Aggregates, at XXXIII-a Conferinta Nationala de Chimie, Caciulata, October 1st-3rd 2014.
-
Eszter Lakatos, Niculina D. Hadade, AnamariaTerec, Ion Grosu: Synthesis and Characterization of some New Pyrene Derivatives - prezentare orala Zilele Academice Iesene, September, 24-26 2015, Iasi.
-
Prof. dr. Ion Grosu - plenary lecture: Self-assembled Supramolecular Architectures, Innovative Host Molecules and Porous Polymers; at A XXXIII-a Conferinta Nationala de Chimie, Caciulata, October 4th-7th 2016.
-
Cosmin V. Crisan, Niculina D. Hadade, Ion Grosu: poster, Active Metal Template Approach in the Synthesis of new Cryptand-Macrocycle Supramolecular Assemblies; ECHC 2016, XXVII European Colloquium on Heterocyclic Chemistry, Amsterdam, The Netherlands - July 3rd - 6th, 2016.
-
The yearly scientific reports containing experimental details of unpublished compounds data can be provided for project evaluation purposes only. To obtain copies of these reports please contact Prof. Dr. Ion Grosu by e-mail at igrosu (at) chem.ubbcluj.ro .