Project PN-II-ID-JRP-RO-FR-2012-0088:
Project timespan
Project team
Abstract
Project objectives
Budget
Results
Title: Dynamic Biomaterials with Multivalent Recognition Properties (DYNMULTIREC)
Romanian Partner: Babes-Bolyai University, Center of Supramolecular Organic and Organometallic Chemistry Project manager: Prof. Dr. Ion Grosu ResearcherID: B-8088-2011
Team:
- Dr. Elena Bogdan ResearcherID: F-1379-2011
- Dr. Niculina D. Hădade ResearcherID: F-9489-2011
- MSc Rednic Monica Irina
- MSc Cosmin Crişan
- MSc Lakatos Eszter
- BSC Ana-Maria Casian
Project timespan
2014 -- 2016
2014 -- 2016
Abstract
The targets of the project Dynamic Biomaterials with Multivalent Recognition Properties (DYNMULTIREC) are focused on:
i) the design, fabrication, investigation of properties and applications of highly selective membranes containing directional channels of variable sizes and binding abilities and exhibiting specific and selective permeability for ions (transition metals and lanthanides) and molecules (peptides, modified aminoacids and sugars, aromatic compounds, water).
ii) the design and use of the dynamic constitutional polymers (dynamers) functionalized with different biologically relevant moieties (i.e. metallochelating ligands or activity-based probes) for oligomerisation of His-tagged proteins and the synthesis of new protein-decorated polymers.
The construction of the membranes (organic-inorganic networks) and of the biodynamers takes into account the following directions:
The targets of the project Dynamic Biomaterials with Multivalent Recognition Properties (DYNMULTIREC) are focused on:
i) the design, fabrication, investigation of properties and applications of highly selective membranes containing directional channels of variable sizes and binding abilities and exhibiting specific and selective permeability for ions (transition metals and lanthanides) and molecules (peptides, modified aminoacids and sugars, aromatic compounds, water).
ii) the design and use of the dynamic constitutional polymers (dynamers) functionalized with different biologically relevant moieties (i.e. metallochelating ligands or activity-based probes) for oligomerisation of His-tagged proteins and the synthesis of new protein-decorated polymers.
The construction of the membranes (organic-inorganic networks) and of the biodynamers takes into account the following directions:
- Adaptive multifunctional membranes based on the incorporation of macrocycles by directed self-assembly into inorganic networks. The challenging idea at this point is the building of the target adaptive multifunctional membranes applying the principles of constitutional dynamic chemistry (CDC).
- Multifunctional organic-inorganic membranes having macrocycles within the pores built by the 2D self-assembly of sterically compatible aromatic units decorated with complementary multiple H-donor / acceptor groups followed by the 3D self-assembly of these macrocycles and their incorporation into organic-inorganic networks via supramolecular contacts (stacking). The main advantages of this approach consist in the fact that one can obtain a set of appropriately decorated aromatic units which then can be assembled in different macrocycles by simply changing the partners belonging to the same set or, like in the previous case, the shape of the self-assembled macrocycles can be induced and amplified by the molecule which is targeted for separation via host-guest interactions.
- Hybrid networks with channels exhibiting highly selective permeability for biomolecules (aminoacids, peptides and modified sugars) based on cyclic peptides made of (LD)n or (LLLD)n sequences. The (LD)n or LLLD)n sequences ensure in one case a quite planar shape of the macrocycles and on the other case [for the LLLD)n sequence] that every forth aminoacid has the side chain oriented inside the macrocycle and they can participate in a decisive way to the selectivity of the membrane for different targets. Furthermore, these membranes can be used for enantiomers separation.
- The development of new biodynamers as advanced synthetic materials for protein immobilization, protein clustering and multivalent interactions with biological modulators is based on the principle of CDC. To build the Dynamic Constitutional Libraries (DCLs) we turned our sights to the acylhydrazone exchange reaction using a set of di- and tri-aldehydes and acylhyrazones decorated with metallochelating, activity-based probes or proteins.
Objectives
1. Design and synthesis of multifunctional directional channels of variable sizes and binding abilities in order to obtain highly selective membranes allowing specific transport of ions and molecules. The channels are built up by the self-assembly of covalent-type macrocycles or of supramolecular macrocycles formed by H-bonds between well-defined complementary partners exhibiting multiple H-donor/acceptor units. The building of the macrocycles uses the principles of the constitutional dynamic chemistry and the sizes and the shapes of the macrocycles will be correlated with the amplification properties of the target ions or molecules for which the membranes has to show permeability.
2. Design and synthesis of multifunctional membranes based on organic-inorganic networks in which the channels are built using cyclic peptides with (LD)n or (LLLD)n sequences. Cyclic peptides with (LD)n sequences exhibit quite planar cycles and all side chains of the aminoacids are oriented outside the cavity. The appropriate decoration of the D aminoacids (especially in the (LLLD)n sequence, in which each forth aminoacid has the side chain oriented inside the cavity) can ensure the high selectivity of the membranes and the efficient transport of biomolecules and the development of applications in biomedical domain and in racemics resolution.
3. Use of the dynamic constitutional polymers (dynamers) functionalised with different biologically relevant moieties (i.e. metallochelating ligands or activity-based probes) for oligomerisation of His-tagged proteins and synthesis of new protein-decorated polymers.
1. Design and synthesis of multifunctional directional channels of variable sizes and binding abilities in order to obtain highly selective membranes allowing specific transport of ions and molecules. The channels are built up by the self-assembly of covalent-type macrocycles or of supramolecular macrocycles formed by H-bonds between well-defined complementary partners exhibiting multiple H-donor/acceptor units. The building of the macrocycles uses the principles of the constitutional dynamic chemistry and the sizes and the shapes of the macrocycles will be correlated with the amplification properties of the target ions or molecules for which the membranes has to show permeability.
2. Design and synthesis of multifunctional membranes based on organic-inorganic networks in which the channels are built using cyclic peptides with (LD)n or (LLLD)n sequences. Cyclic peptides with (LD)n sequences exhibit quite planar cycles and all side chains of the aminoacids are oriented outside the cavity. The appropriate decoration of the D aminoacids (especially in the (LLLD)n sequence, in which each forth aminoacid has the side chain oriented inside the cavity) can ensure the high selectivity of the membranes and the efficient transport of biomolecules and the development of applications in biomedical domain and in racemics resolution.
3. Use of the dynamic constitutional polymers (dynamers) functionalised with different biologically relevant moieties (i.e. metallochelating ligands or activity-based probes) for oligomerisation of His-tagged proteins and synthesis of new protein-decorated polymers.
Budget
| Budget chapter (expenses) | 2014 (lei) | 2015 (lei) | 2016 (lei) | Total | |
|---|---|---|---|---|---|
| 1 | Salaries | 200.000 | 200.000 | 210.000 | 610.000 |
| 2 | Overhead | 40.000 | 30.000 | 32.272,72 | 102.272,72 |
| 3 | Mobility | 25.000 | 25.000 | 31.000 | 81.000 |
| 4 | Logistics | 175.000 | 75.000 | 81.727,28 | 331.727,28 |
| 5 | Total | 440.000 | 330.000 | 355.000 | 1.125.000 |
Results 2014
Dynamic complexes of a tripyridinic-diimino ligand were investigated in collaboration between the team of Montpellier and the team of Cluj.1 The complexes of the library (formed in the presence of several cations) are shown in Scheme 1.
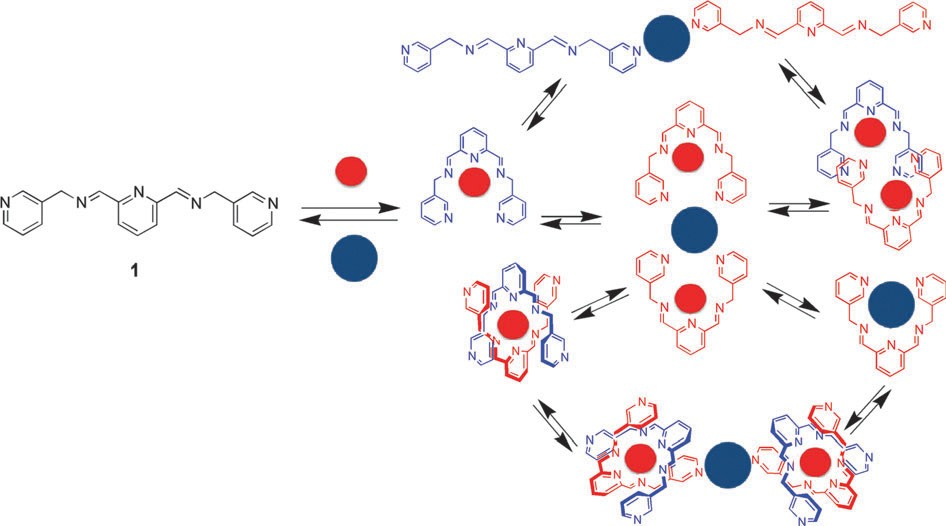
Scheme 1
The equilibria are shifted towards one or other of structures in correlation with the preference for different cations. Structure a is obtained with Zn2+, b with Ag+, while structures c and d are obtained in the presence of Cu2+ or Pb2+, respectively (Scheme 2).
Scheme 2
A literature investigation was carried out in order to evaluate the data reported concerning the macrocycles with different aromatic heterocyclic units. The results of this investigation was fructified by publishing a review paper in J. Incl. Phenom. Macrocycl. Chem.2
Acyl-hydrazones and derivatives of the nitriloacetic acid, requested by the third objective were obtained in agreement with the reactions shown in Schemes 3 and 4.
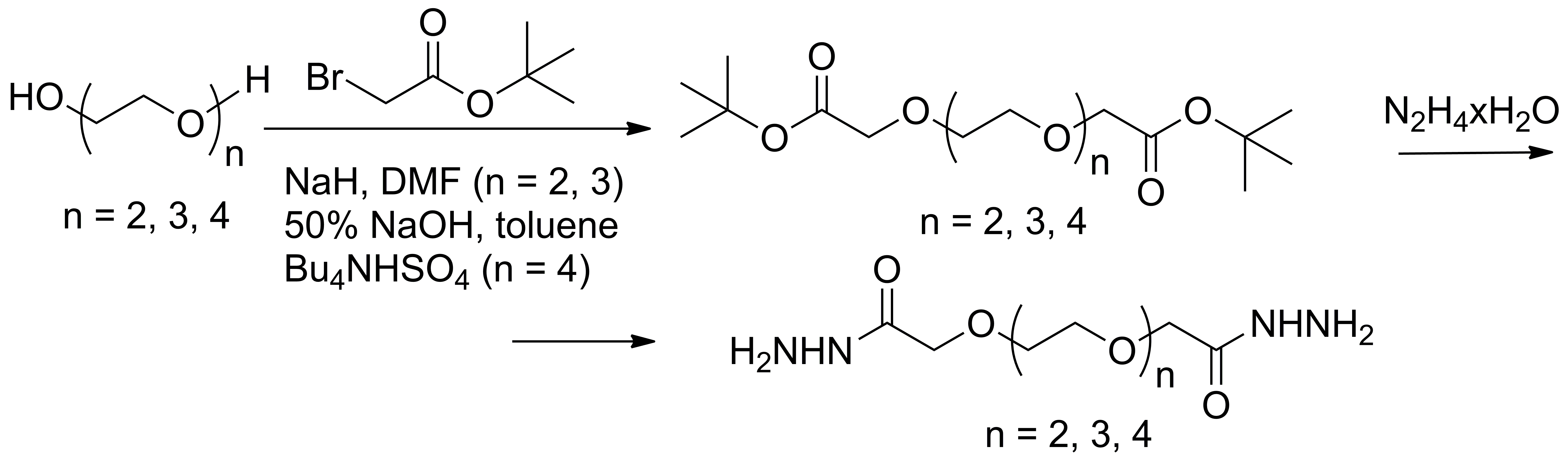
Scheme 3
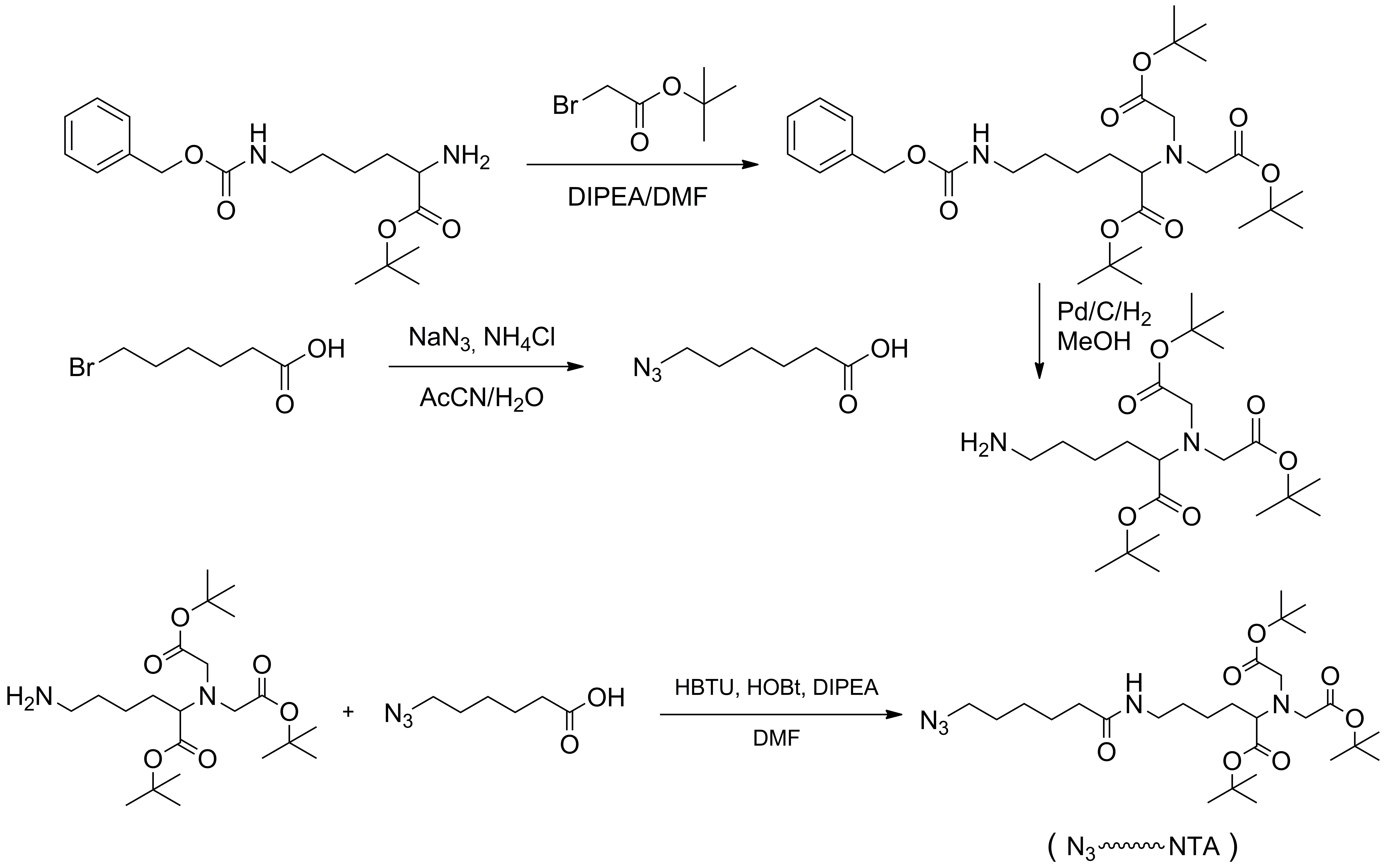
Scheme 4
For objective 1 some derivatives of triphenylamine, and expanded triphenylamine (see Scheme 5) were obtained in good yields. The studies concerning their self organization are running.

Scheme 5
For objective 2 we carried out the synthesis of cyclic peptides with octa and- dodeca aminoacids in the cycle. Symmetrically two of the amino acids of the cycle are decorated either with etynyl or azide groups. In the next future the click reaction will be performed in order to obtain the cryptands with cyclopeptides as reference units.
1. Kocsis, I., Dumitrescu, D., Legrand, Y.-M., Van Der Lee, A., Grosu, I., Bărboiu, M., Self-sorting of dynamic metallosupramolecular libraries (DMLs) via metal-driven selection, ChemComm., 2014, 50, 2621.
2. Rednic, M. I.; Hădade, N. D.; Bogdan, E.; Grosu, I., Macrocycles Embedding Phenothiazine or Similar Heterocycles, J. Incl. Phenom. Macrocycl. Chem., 2014, DOI 10.1007/s10847-014-0455-x.
Dissemination
Papers
ReportsDynamic complexes of a tripyridinic-diimino ligand were investigated in collaboration between the team of Montpellier and the team of Cluj.1 The complexes of the library (formed in the presence of several cations) are shown in Scheme 1.

Scheme 1
The equilibria are shifted towards one or other of structures in correlation with the preference for different cations. Structure a is obtained with Zn2+, b with Ag+, while structures c and d are obtained in the presence of Cu2+ or Pb2+, respectively (Scheme 2).

Scheme 2
A literature investigation was carried out in order to evaluate the data reported concerning the macrocycles with different aromatic heterocyclic units. The results of this investigation was fructified by publishing a review paper in J. Incl. Phenom. Macrocycl. Chem.2
Acyl-hydrazones and derivatives of the nitriloacetic acid, requested by the third objective were obtained in agreement with the reactions shown in Schemes 3 and 4.

Scheme 3

Scheme 4
For objective 1 some derivatives of triphenylamine, and expanded triphenylamine (see Scheme 5) were obtained in good yields. The studies concerning their self organization are running.

Scheme 5
For objective 2 we carried out the synthesis of cyclic peptides with octa and- dodeca aminoacids in the cycle. Symmetrically two of the amino acids of the cycle are decorated either with etynyl or azide groups. In the next future the click reaction will be performed in order to obtain the cryptands with cyclopeptides as reference units.
1. Kocsis, I., Dumitrescu, D., Legrand, Y.-M., Van Der Lee, A., Grosu, I., Bărboiu, M., Self-sorting of dynamic metallosupramolecular libraries (DMLs) via metal-driven selection, ChemComm., 2014, 50, 2621.
2. Rednic, M. I.; Hădade, N. D.; Bogdan, E.; Grosu, I., Macrocycles Embedding Phenothiazine or Similar Heterocycles, J. Incl. Phenom. Macrocycl. Chem., 2014, DOI 10.1007/s10847-014-0455-x.
Results 2015
In 2015 the research activities on the project Dynamic Biomaterials with Multivalent Recognition Properties (DYNMULTIREC) was focused on the objectives G1-G5.
For the objective G3, new triphenylamines decorated with multiple H-donor and H-acceptor groups were obtained in large amounts which allow their investigation in membranes (Schemes 1-3).
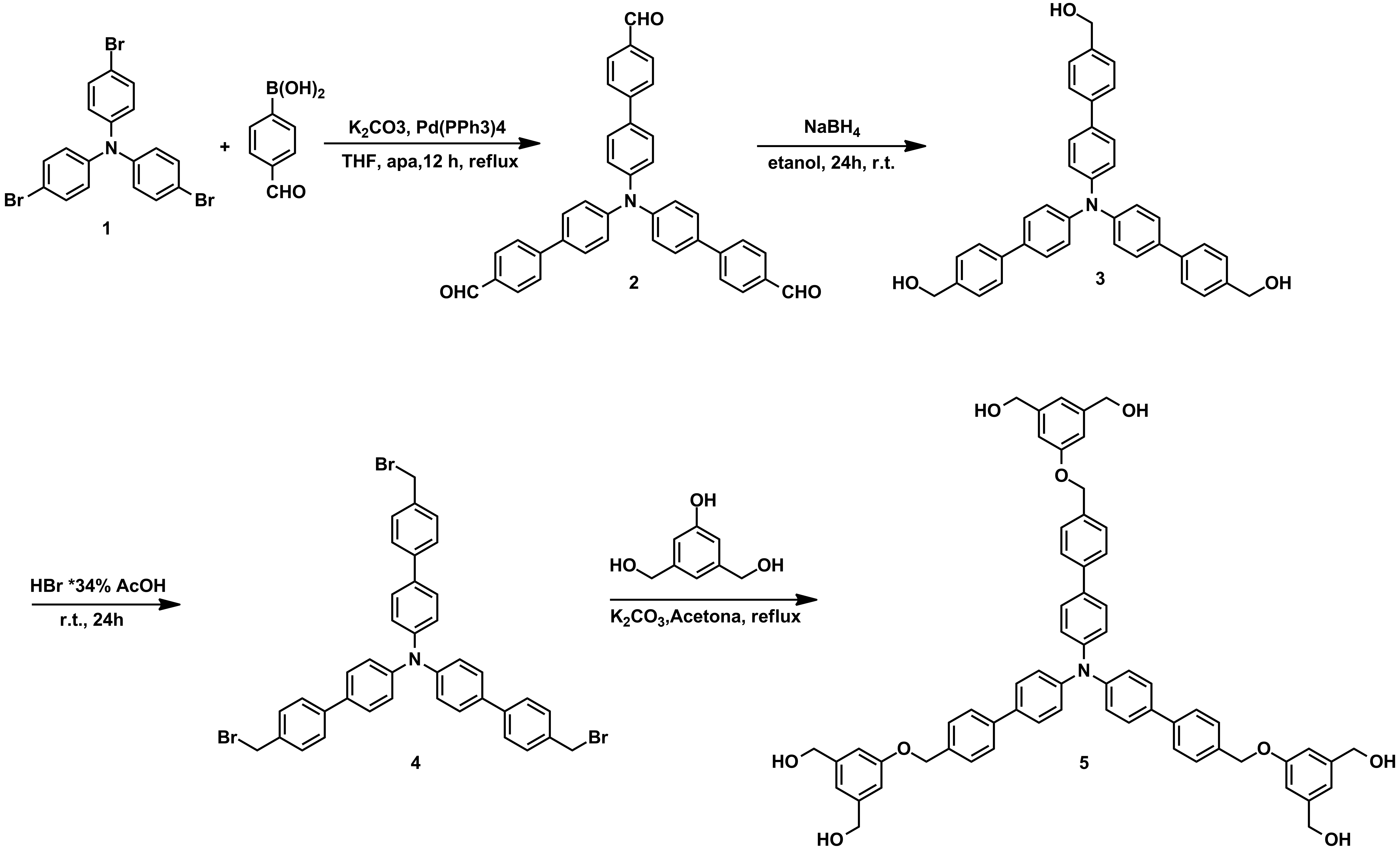
Scheme 1
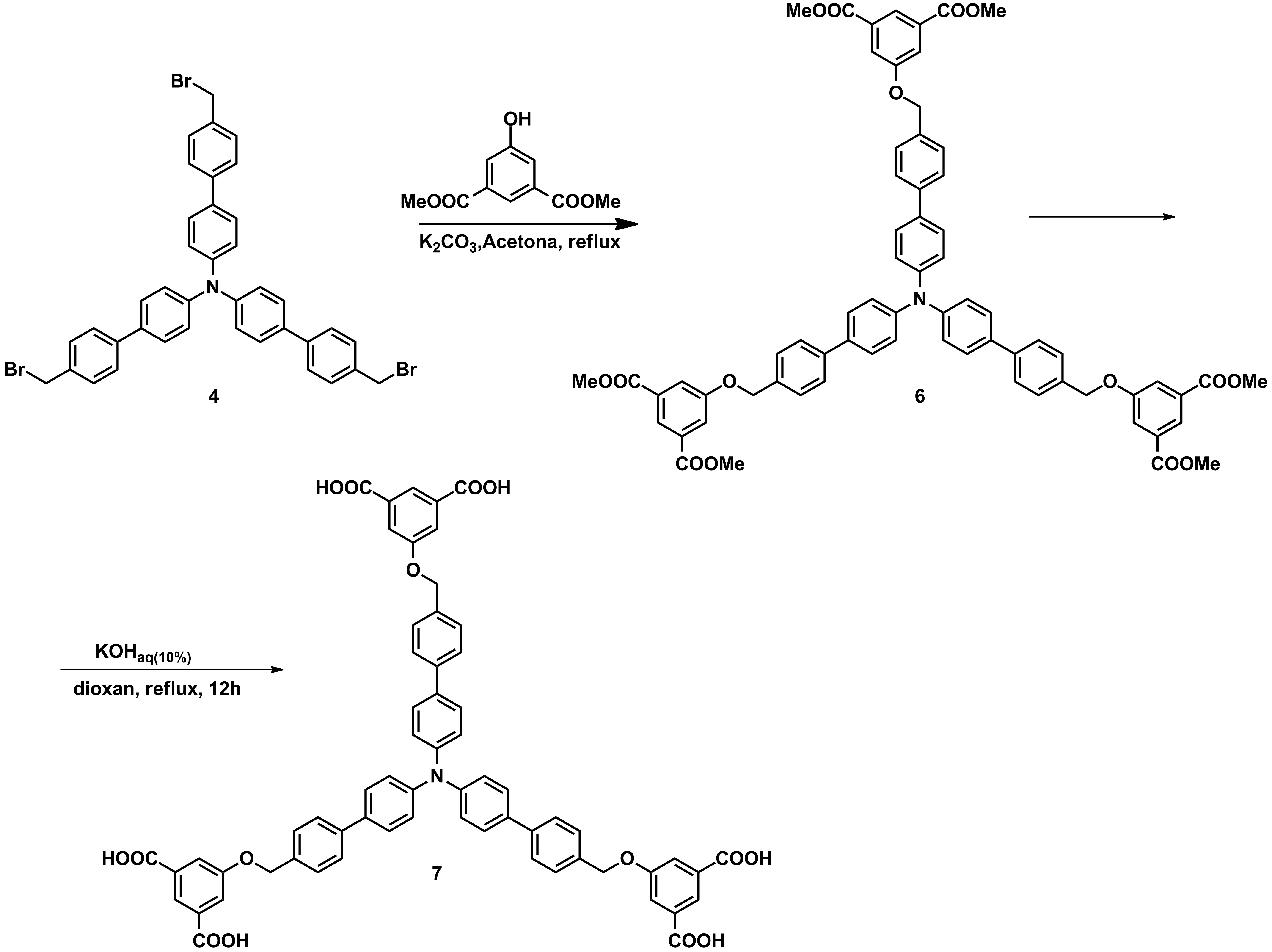
Scheme 2
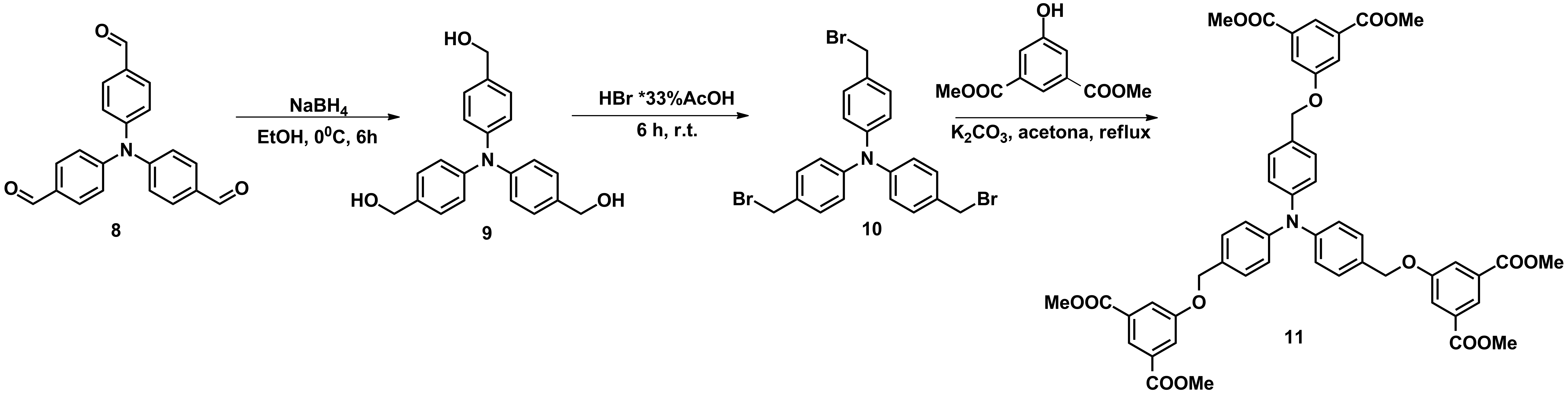
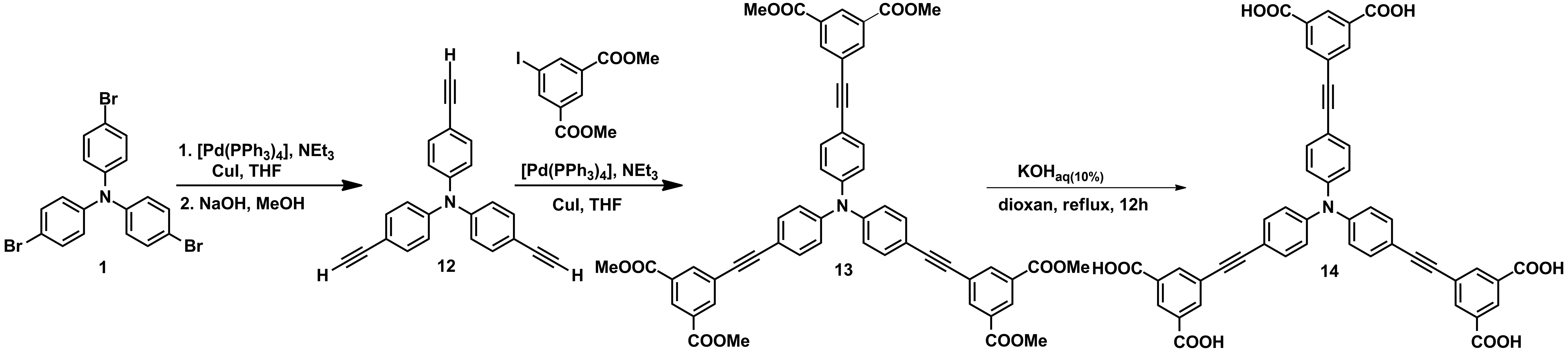
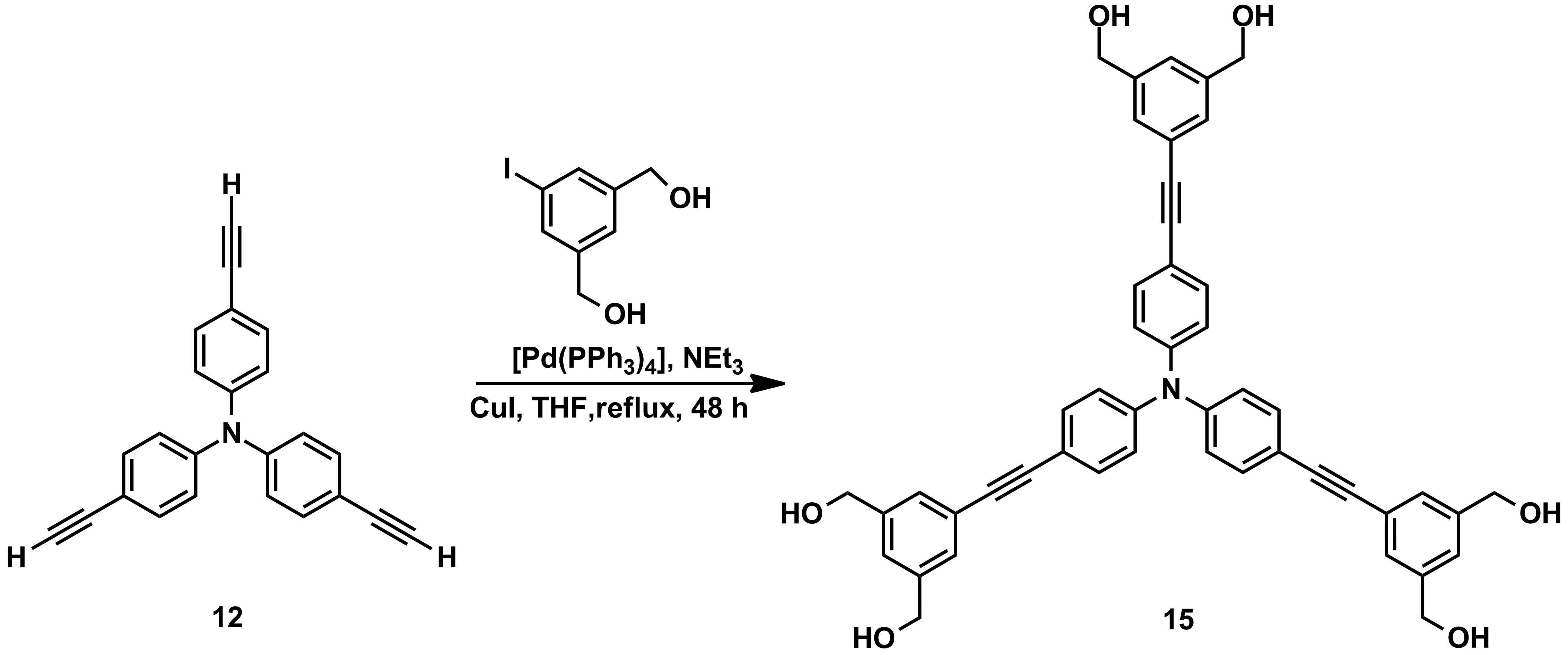
Scheme 3
For this objective some derivatives of triphenylamine decorated with cationic groups derived from amino-guanidine or the hydrazide of N,N,N-trimethylglycine (16, 17; Scheme 4) were also obtained.
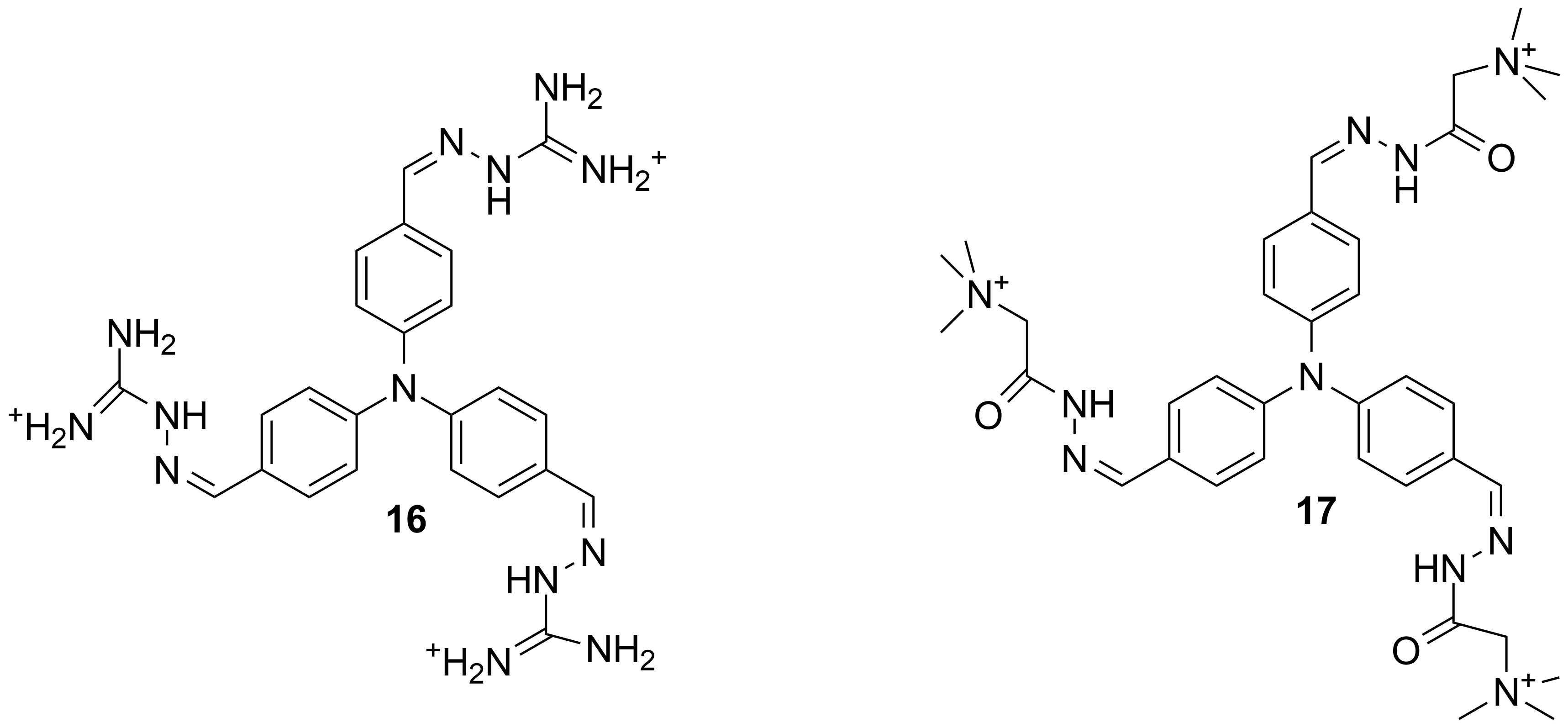
Scheme 4
These cations have the ability to self assemble and to form under photochemical conditions organic conductor or to interact specifically with DNA sequences leading to the recognition of several patterns.
The self-assembly of 16 and 17 lead to chiral structures which can be investigated via CD experiments. The CD diagrams reveal significant modifications in the presence of some ss-DNA sequences. These modifications suggest a change of the helicity of the DNA sequences during their interaction with 16 and 17. For details see Chem. Commun, 2016, DOI: 10.1039/C5CC08283H.
For G1 a package of several di and trialdehydes as well as di and triamines were made up (Scheme 5). With these compounds dynamic libraries will be obtained and the amplifications of the reactions will be tested with several templates firstly in chemical systems then in membranes. Some of these compounds are commercial others were prepared in our laboratory.
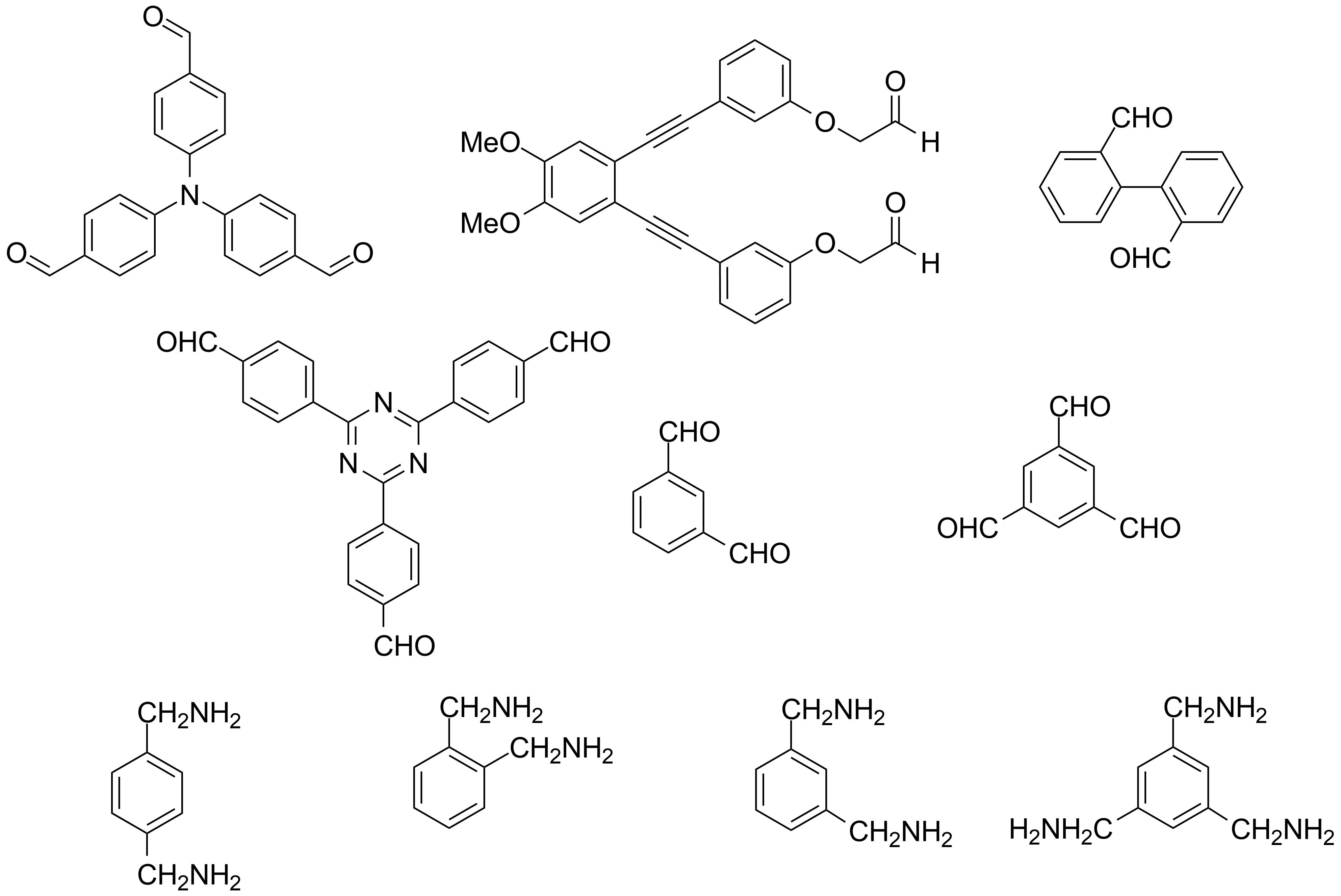
Scheme 5
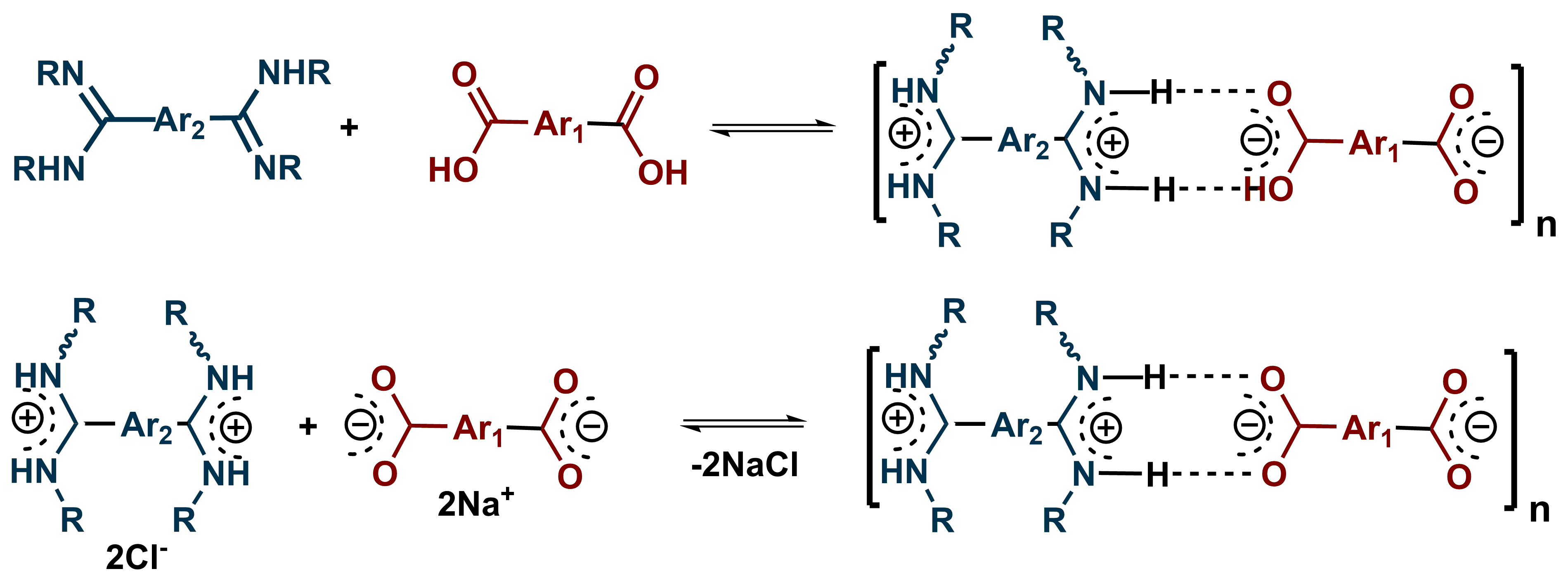
For G2 we focused on compounds which can form "salt-bridges" and where the classic H-bonds are doubled by favorable interactions between the separated positive and negative charges. We investigated the associations among diamidines and dicarboxylic and disulfonic aromatic acids. The results are very interesting and a paper is in preparation.
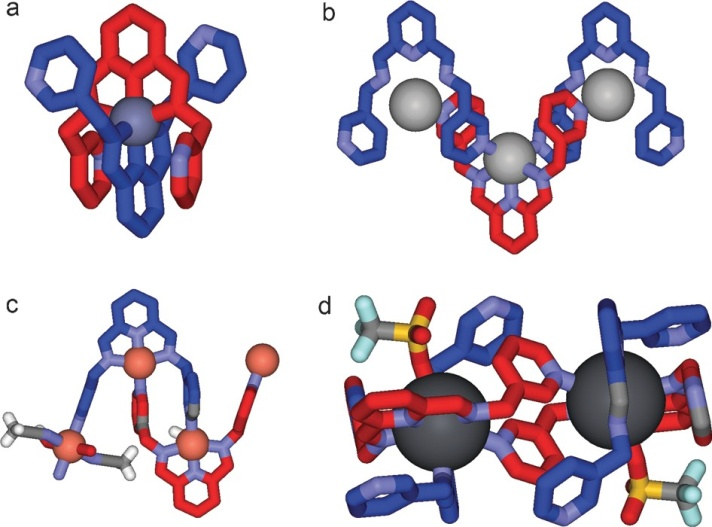
In another direction connected to the same G2 objective we were interested in the adaptative self-assembly of compounds which can generate large cavities. The investigated compounds were designed initially for other applications (as MOFs and COFs), but some of them could be crystallized and could be investigated by single crystal X-ray diffractometry (Scheme 6).
Scheme 6
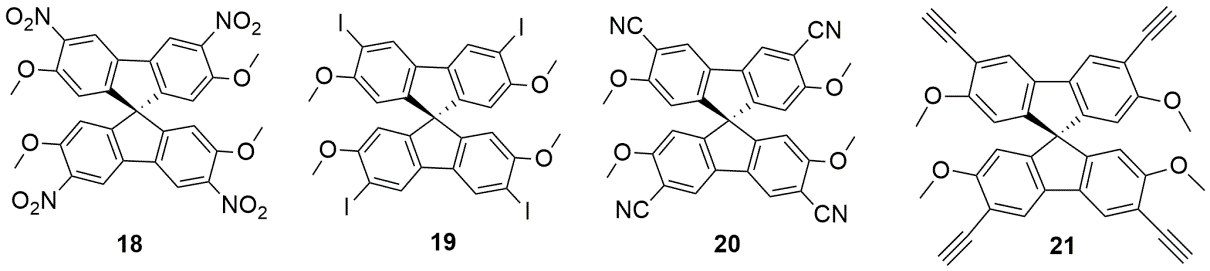
The results of the X ray diffractometry investigations are shown in Figures 1-4.
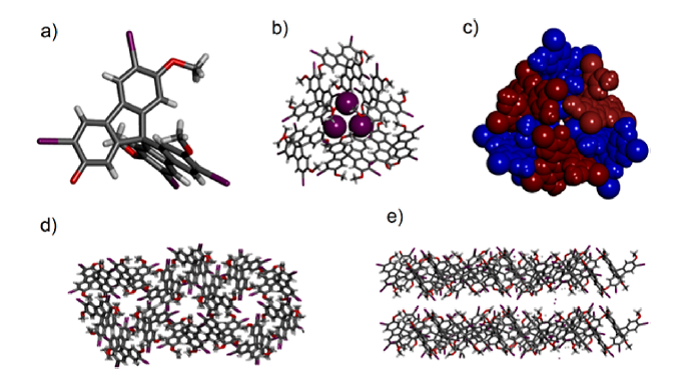
Figure 1. Molecular structure (a) of synton 18 and of its hexameric association 186 in stick (b) and CPK (c) representations; views of the lattice top (d) and from lateral (e)
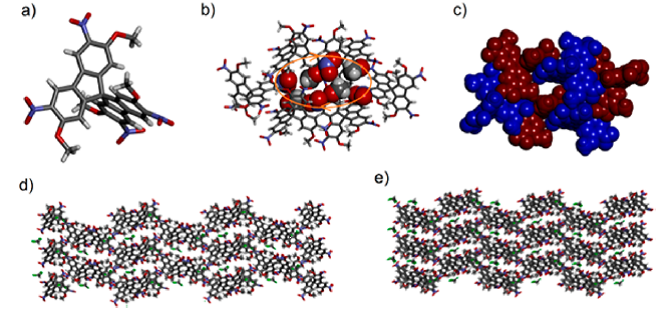
Figure 2. Molecular structure (a) of synton 19 and of its hexameric association 196 in stick (b) and CPK (c) representations; views of the lattice top (d) and from lateral (e)
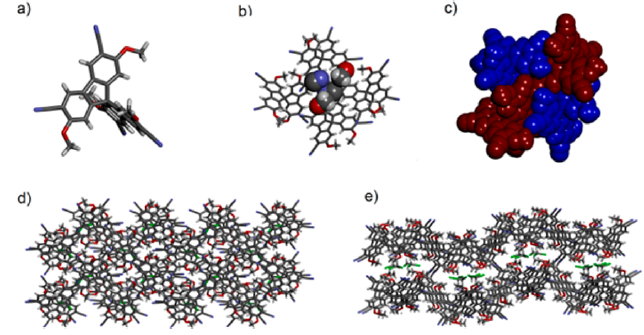
Figure 3. Molecular structure (a) of synton 20 and of its hexameric association 206 in stick (b) and CPK (c) representations; views of the lattice top (d) and from lateral (e)

Figure 4. Molecular structure (a) of synton 21 and of its hexameric association 216 in stick (b) and CPK (c) representations; views of the lattice top (d) and from lateral (e)
These results were published in Organic Letters (2015).
At G5 as a continuation of the previously reported data we got the access to hydrazides decorated with NTA units which can be incorporated in dynamers (Scheme 7).
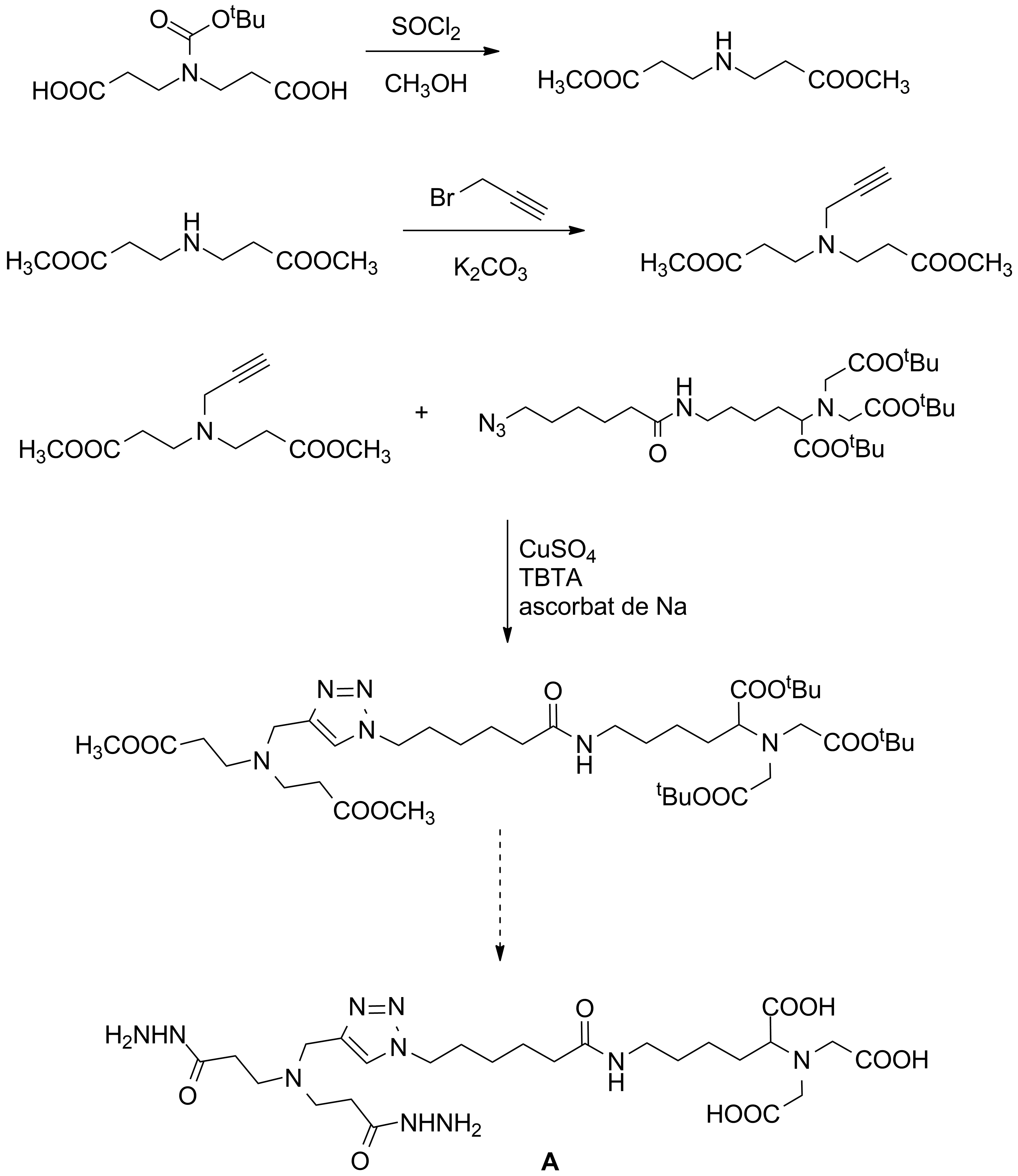
Scheme 7
In 2015 the research activities on the project Dynamic Biomaterials with Multivalent Recognition Properties (DYNMULTIREC) was focused on the objectives G1-G5.
For the objective G3, new triphenylamines decorated with multiple H-donor and H-acceptor groups were obtained in large amounts which allow their investigation in membranes (Schemes 1-3).

Scheme 1

Scheme 2



Scheme 3
For this objective some derivatives of triphenylamine decorated with cationic groups derived from amino-guanidine or the hydrazide of N,N,N-trimethylglycine (16, 17; Scheme 4) were also obtained.

Scheme 4
These cations have the ability to self assemble and to form under photochemical conditions organic conductor or to interact specifically with DNA sequences leading to the recognition of several patterns.
The self-assembly of 16 and 17 lead to chiral structures which can be investigated via CD experiments. The CD diagrams reveal significant modifications in the presence of some ss-DNA sequences. These modifications suggest a change of the helicity of the DNA sequences during their interaction with 16 and 17. For details see Chem. Commun, 2016, DOI: 10.1039/C5CC08283H.
For G1 a package of several di and trialdehydes as well as di and triamines were made up (Scheme 5). With these compounds dynamic libraries will be obtained and the amplifications of the reactions will be tested with several templates firstly in chemical systems then in membranes. Some of these compounds are commercial others were prepared in our laboratory.

Scheme 5
For G2 we focused on compounds which can form "salt-bridges" and where the classic H-bonds are doubled by favorable interactions between the separated positive and negative charges. We investigated the associations among diamidines and dicarboxylic and disulfonic aromatic acids. The results are very interesting and a paper is in preparation.
In another direction connected to the same G2 objective we were interested in the adaptative self-assembly of compounds which can generate large cavities. The investigated compounds were designed initially for other applications (as MOFs and COFs), but some of them could be crystallized and could be investigated by single crystal X-ray diffractometry (Scheme 6).

Scheme 6
The results of the X ray diffractometry investigations are shown in Figures 1-4.

Figure 1. Molecular structure (a) of synton 18 and of its hexameric association 186 in stick (b) and CPK (c) representations; views of the lattice top (d) and from lateral (e)

Figure 2. Molecular structure (a) of synton 19 and of its hexameric association 196 in stick (b) and CPK (c) representations; views of the lattice top (d) and from lateral (e)

Figure 3. Molecular structure (a) of synton 20 and of its hexameric association 206 in stick (b) and CPK (c) representations; views of the lattice top (d) and from lateral (e)

Figure 4. Molecular structure (a) of synton 21 and of its hexameric association 216 in stick (b) and CPK (c) representations; views of the lattice top (d) and from lateral (e)
These results were published in Organic Letters (2015).
At G5 as a continuation of the previously reported data we got the access to hydrazides decorated with NTA units which can be incorporated in dynamers (Scheme 7).

Scheme 7
Results 2016
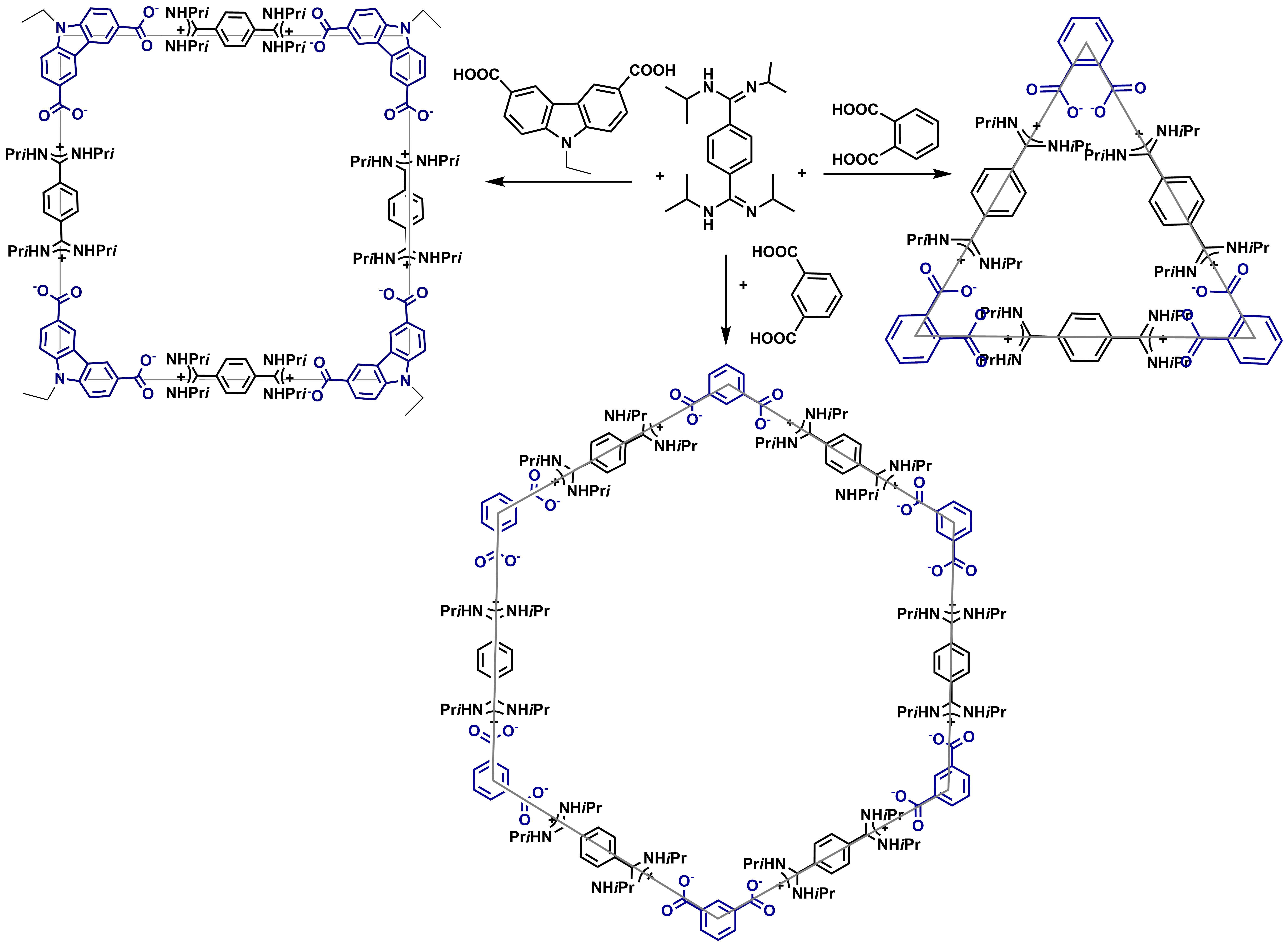
For G2 objective we investigated several supramolecular architectures based on "salt-bridge" interactions. We targeted to obtain triangles, squares and hexagons by the appropriate selection of the reacting molecules (Scheme 1).
We used benzenediamidines (para) and several dicarboxylic and disulfonic aromatic acids (Table 1). The syntheses are shown in Scheme 2.
The investigations were carried out both in solution and solid state. In solid state we observed a strong competition among the formation of salt-bridges and simple H-bonds (including the participation of water molecules). We obtained 8 molecular structures using single crystal X-ray diffractometry which reveal spectacular supramolecular associations (Figura 1).
Scheme 1
Scheme 2
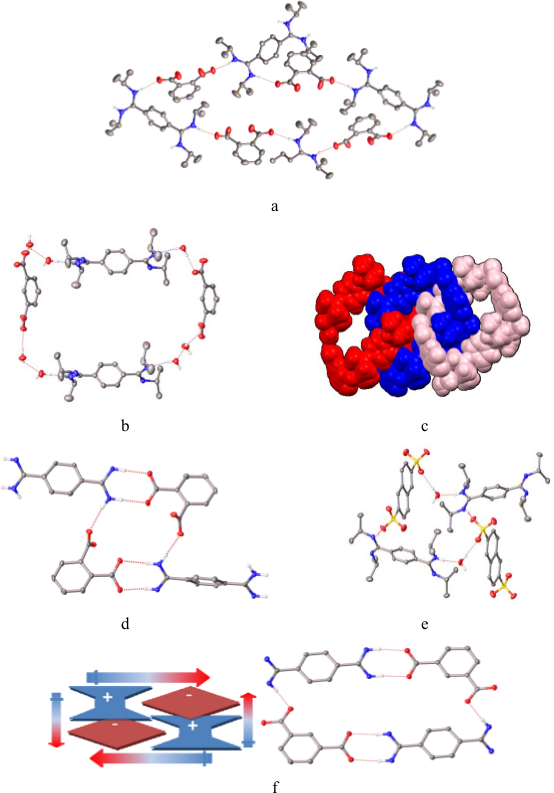
Figure 1. Details of the associations in 1@3 (a), 1@4 (b,c), 1@8 (d), 2@3 (e) si 2@4 (f).
For this task the complexation of β-HCH (Scheme 3) with different anions (Cl-, Br-, I- and HSO4-) was studied.
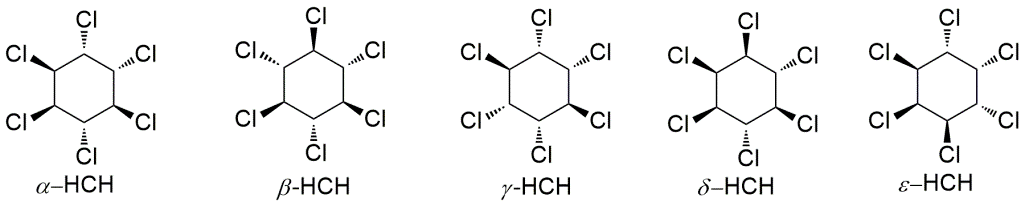
Scheme 3. HCH diastereoisomers.
Monocrystals suitable for X-ray diffractometru were obtained using the complexes of β-HCH with PyxHCl and PyxHBr and for β-HCH itself. β-HCH exhibit a cubic form of high symmetry (Figure 2).
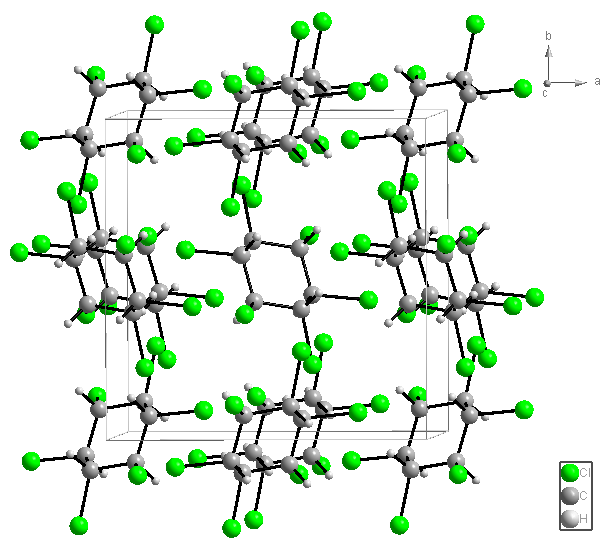
Figure 2. Molecular structure of β-HCH.
The following molecular combinations were obtained: C6H6Cl6x2C5H5N+-Hx2Cl- (A), C6H6Cl6x2C5H5N+-Hx2Br- (B) and C6H6Cl6xC5H5NxC5H5N+-HxBr- (C). The relevant units (Figure 3) for A and B present two anions on the C3 axis of β-HCH. Each unit reveals 6 CH (axial) / X- connections (dCl(-)- - - HC(sp3) = 2.866, 2.866 and 2.710 Å for A; dBr(-)- - - HC(sp3) = 2.965, 2.965 and 2.847 Å for B). The formation of supramolecular polymers is shown in Figure 4.
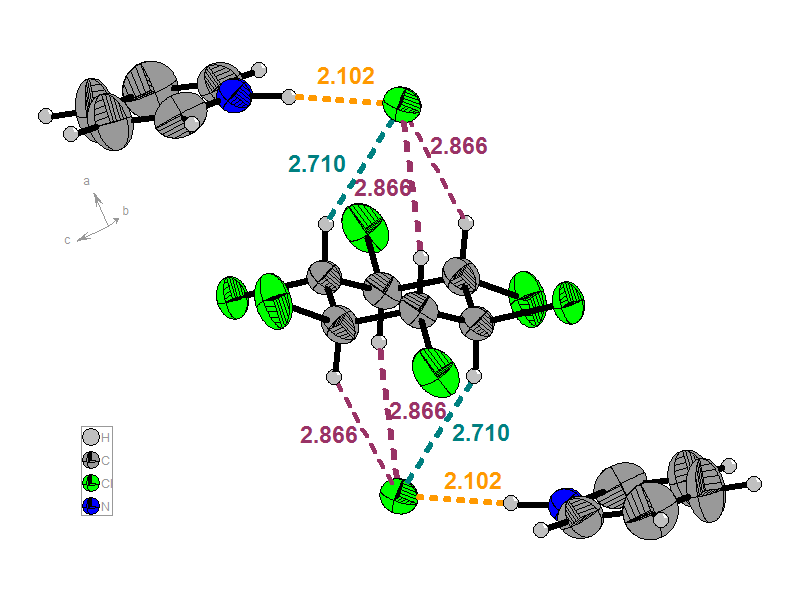
a)
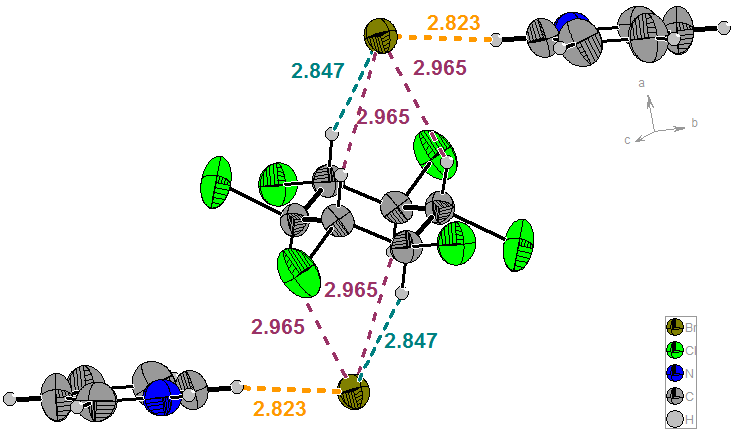
b)
Figure 3. Repetitive units for A (a) and B (b)
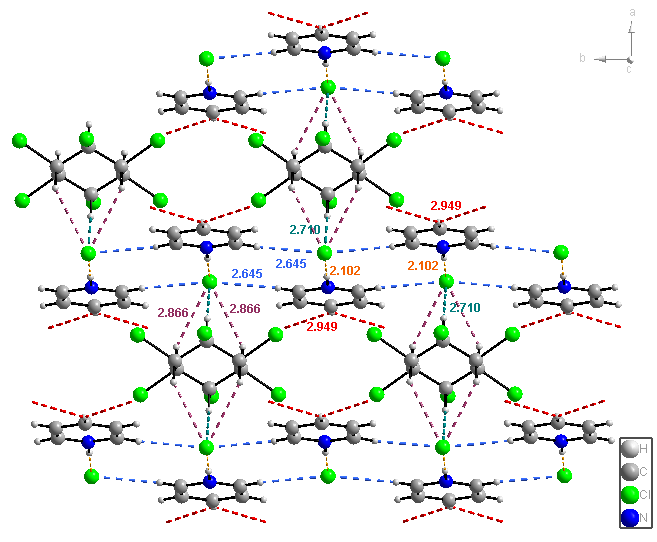
Figure 4. View of the lattice of A (similar to B).
In the case of C, two pyridine molecules are connected via a proton and form a pyridinium-pyridine cation and the pattern forms by two Br- anions and the β-HCH ring is also present (Figure 5). In the lattice of C (Figures 6 and 7) β-HCH and Br- form spectacular columns. These columns form a 2D network by halogen bonding.
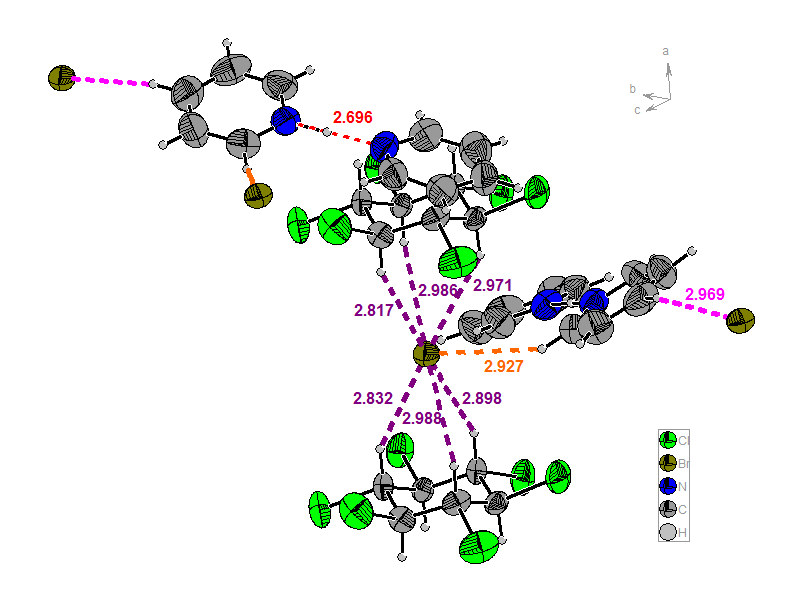
Figure 5. Representation of the repetitive unit for C.
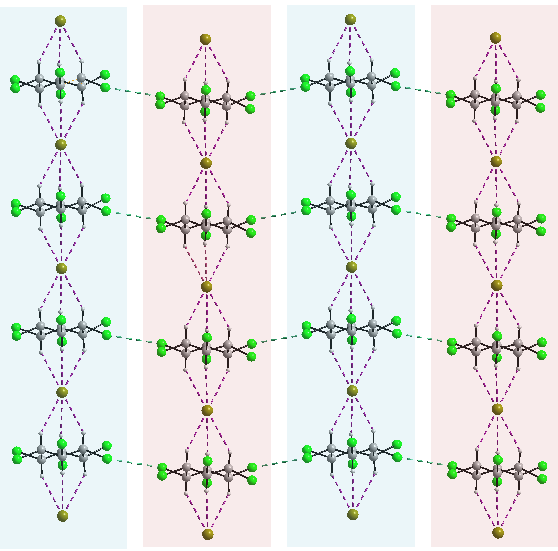
Figure 6. 2D network for C.
The connections of pyridinium-pyridine units with Br- ensure the 3D structure of the supramolecular polymer (Figure 7).
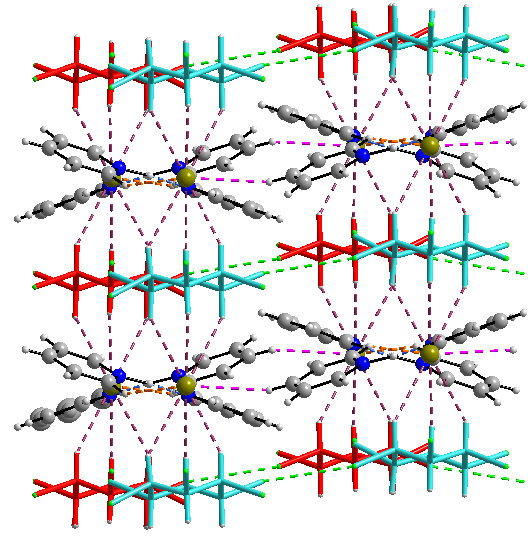
Figure 7. 3D network for C.
The comparison of the columns of C with the famous endless column of Brâncusi is shown in Figure 8.
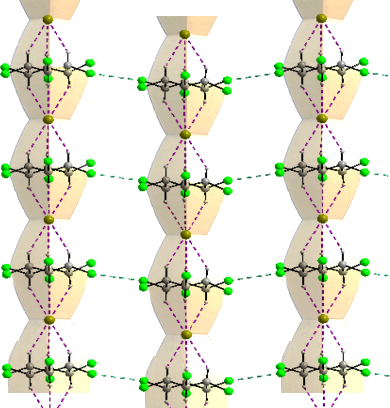
Figure 8. Comparison of C with the endless column of Brâncusi.
The stoichiometry of the β-HCH-anion (Cl-, Br-, I- and HSO4-) complexes in solution were determined by NMR titrations. The JOB-PLOT experiments allowed the determination of the stability constants of these complexes (the values were in the range 1.35 – 2.20 L/molx103).
The formation of the β-HCH-anion (Cl-, Br-, I- and HSO4-) complexes in solution were proved by ESI(-)-HRMS experiments, too. The peaks m/z= 324.8267, 370.7728, 416.7618 and 386.8163 corresponding to [HCH+Cl-], [HCH+Br-], [HCH+I-] and [HCH+HSO4-] species were observed in the investigated spectra (Figures 9 and 10).
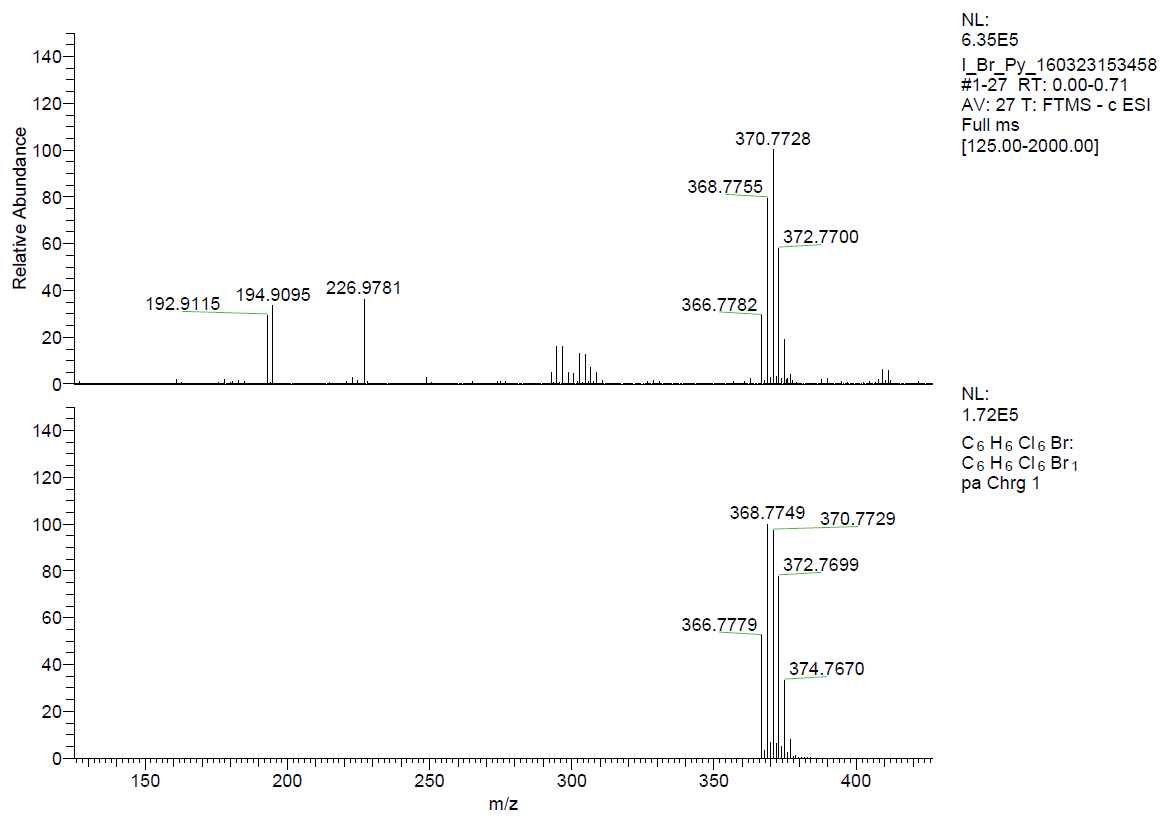
Figure 9. ESI(-)-HRMS experimental (top) and calculated (bottom) spectra of [HCH·Br-].
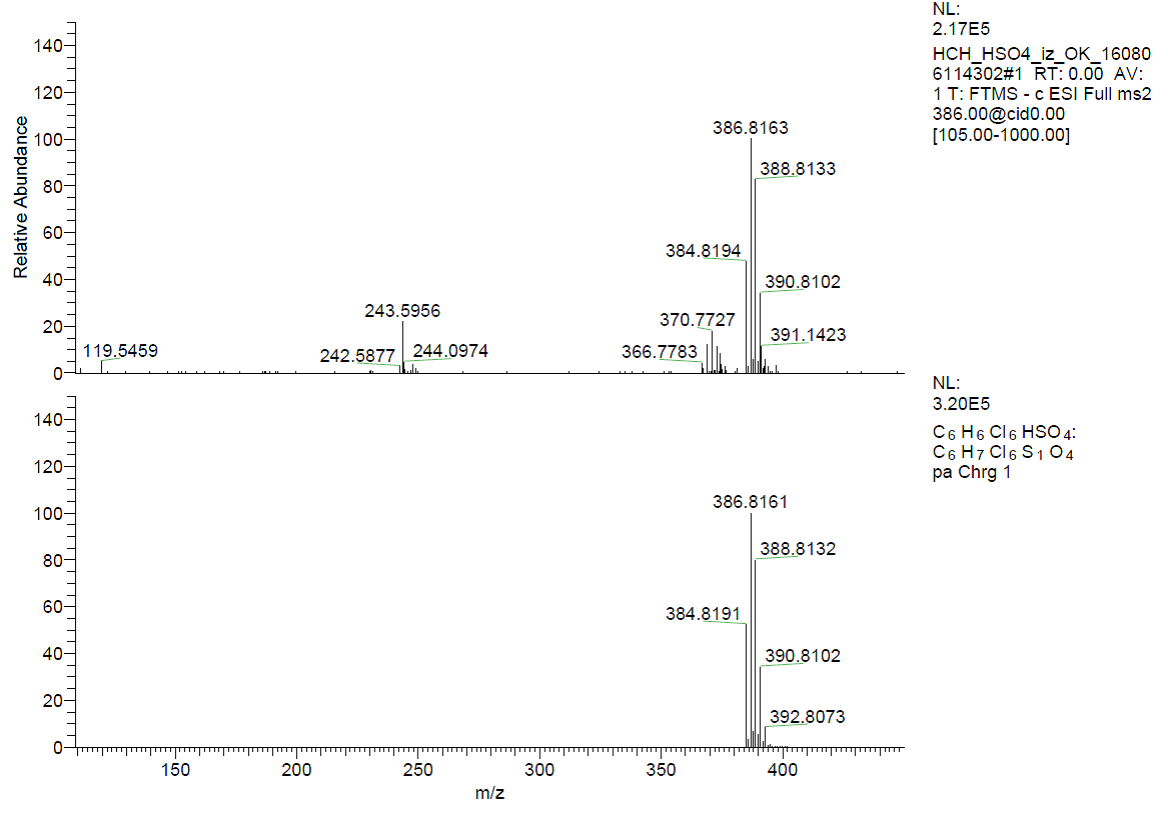
Figure 10. ESI(-)-HRMS experimental (top) and calculated (bottom) spectra of [HSO4-].
For G2 objective we investigated several supramolecular architectures based on "salt-bridge" interactions. We targeted to obtain triangles, squares and hexagons by the appropriate selection of the reacting molecules (Scheme 1).
We used benzenediamidines (para) and several dicarboxylic and disulfonic aromatic acids (Table 1). The syntheses are shown in Scheme 2.
The investigations were carried out both in solution and solid state. In solid state we observed a strong competition among the formation of salt-bridges and simple H-bonds (including the participation of water molecules). We obtained 8 molecular structures using single crystal X-ray diffractometry which reveal spectacular supramolecular associations (Figura 1).

Scheme 1

Scheme 2
Table 1. Diamidines and diacids.
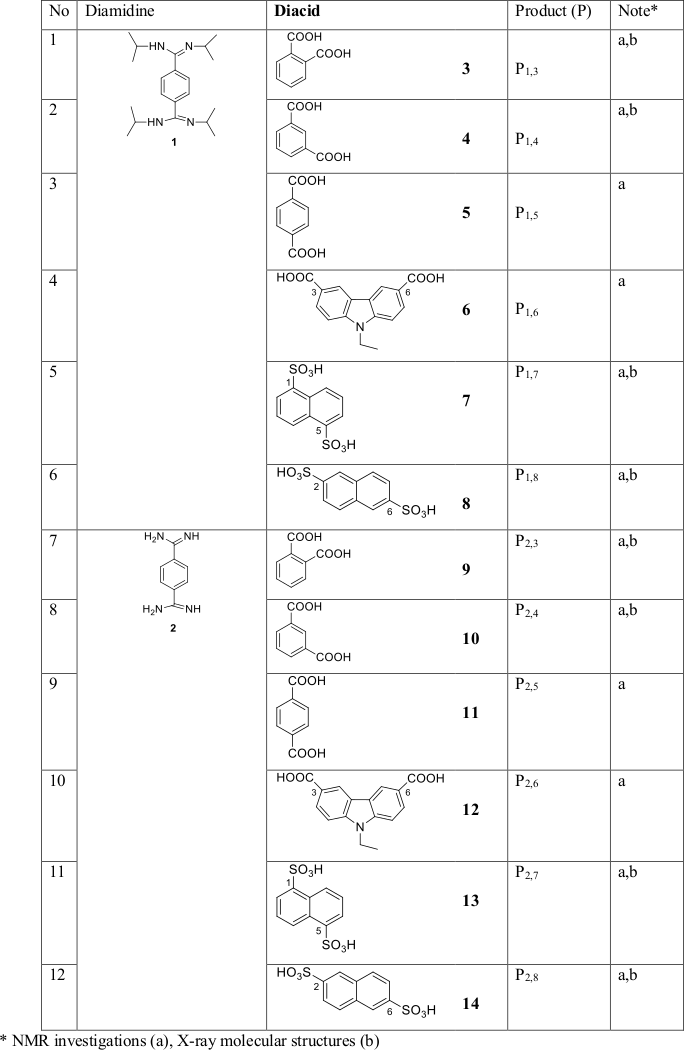


Figure 1. Details of the associations in 1@3 (a), 1@4 (b,c), 1@8 (d), 2@3 (e) si 2@4 (f).
For this task the complexation of β-HCH (Scheme 3) with different anions (Cl-, Br-, I- and HSO4-) was studied.

Scheme 3. HCH diastereoisomers.
Monocrystals suitable for X-ray diffractometru were obtained using the complexes of β-HCH with PyxHCl and PyxHBr and for β-HCH itself. β-HCH exhibit a cubic form of high symmetry (Figure 2).

Figure 2. Molecular structure of β-HCH.
The following molecular combinations were obtained: C6H6Cl6x2C5H5N+-Hx2Cl- (A), C6H6Cl6x2C5H5N+-Hx2Br- (B) and C6H6Cl6xC5H5NxC5H5N+-HxBr- (C). The relevant units (Figure 3) for A and B present two anions on the C3 axis of β-HCH. Each unit reveals 6 CH (axial) / X- connections (dCl(-)- - - HC(sp3) = 2.866, 2.866 and 2.710 Å for A; dBr(-)- - - HC(sp3) = 2.965, 2.965 and 2.847 Å for B). The formation of supramolecular polymers is shown in Figure 4.

a)

b)
Figure 3. Repetitive units for A (a) and B (b)

Figure 4. View of the lattice of A (similar to B).
In the case of C, two pyridine molecules are connected via a proton and form a pyridinium-pyridine cation and the pattern forms by two Br- anions and the β-HCH ring is also present (Figure 5). In the lattice of C (Figures 6 and 7) β-HCH and Br- form spectacular columns. These columns form a 2D network by halogen bonding.

Figure 5. Representation of the repetitive unit for C.

Figure 6. 2D network for C.
The connections of pyridinium-pyridine units with Br- ensure the 3D structure of the supramolecular polymer (Figure 7).

Figure 7. 3D network for C.
The comparison of the columns of C with the famous endless column of Brâncusi is shown in Figure 8.

Figure 8. Comparison of C with the endless column of Brâncusi.
The stoichiometry of the β-HCH-anion (Cl-, Br-, I- and HSO4-) complexes in solution were determined by NMR titrations. The JOB-PLOT experiments allowed the determination of the stability constants of these complexes (the values were in the range 1.35 – 2.20 L/molx103).
The formation of the β-HCH-anion (Cl-, Br-, I- and HSO4-) complexes in solution were proved by ESI(-)-HRMS experiments, too. The peaks m/z= 324.8267, 370.7728, 416.7618 and 386.8163 corresponding to [HCH+Cl-], [HCH+Br-], [HCH+I-] and [HCH+HSO4-] species were observed in the investigated spectra (Figures 9 and 10).

Figure 9. ESI(-)-HRMS experimental (top) and calculated (bottom) spectra of [HCH·Br-].

Figure 10. ESI(-)-HRMS experimental (top) and calculated (bottom) spectra of [HSO4-].
Dissemination
Papers
- Kocsis, I., Dumitrescu, D., Legrand, Y.-M., Van Der Lee, A., Grosu, I., Bărboiu, M., Self-sorting of dynamic metallosupramolecular libraries (DMLs) via metal-driven selection, ChemComm., 2014, 50, 2621-2623.
- Rednic, M. I.; Hădade, N. D.; Bogdan, E.; Grosu, I., Macrocycles Embedding Phenothiazine or Similar Heterocycles, J. Incl. Phenom. Macrocycl. Chem., 2014, DOI: 10.1007/s10847-014-0455-x.
- Pop, L., Dumitru, F., Hadade, N.D., Legrand, Y.-M., Van der Lee, A., Barboiu, M., Grosu, I., Exclusive Hydrophobic Self-Assembly of Adaptive Solid-State Networks of Octasubstituted 9,9'-Spirobifluorenes Org. Lett., 2015, 17, 3494-3497.
- Kocsis, I.; Rotaru, A.; Legrand, Y.-M.; Grosu I.; Barboiu M., Supramolecular rulers enabling selective detection of pure short ssDNA via chiral self-assembly, Chem. Commun, 2015, 52, 386-389. DOI: 10.1039/C5CC08283H.
- Rednic, M. I.; Hadade, N. D.; Bogdan, E; Grosu, I*, Macrocycles embedding phenothiazine or similar nitrogen and/or sulphur containing heterocycles; J. Incl. Phenom. Macrocycl. Chem., 2015, 81, 263-293.
- Pop, L.; Hadade, N. D.; van der Lee, A.; Barboiu, M.; Grosu, I.*; Legrand.; Y.-M.*, Occurence of Charge-Assisted Hydrogen Bonding in Bis-amidine Complexes Generating Macrocycles, Cryst. Growth Des., 2016, 16, 3271–3278.
- Rednic, M. I.; Varga, R. A.; Bende, A.; Grosu, I. G.; Miclaus, M.; Hadade, N. D.; Terec, A.; Bogdan, E.; Grosu, I.*, Supramolecular anion recognition by β-HCH; Chem. Commun., 2016, 52, 12322-12325.
-
Prof. dr. Ion Grosu -keynote lecture, 8-ème Colloque Franco - Roumain de Chimie Appliquée, September 15th - 18th 2014, ENSCM, Montpellier, France.
Macrocycles, Cyclophanes, Cryptandes et Architectures Supramoléculaires Obtenues par Liaisons d'Hydrogène.
-
Ana-Maria Casian, Niculina D. Hadade, Elena Bogdan, Ion Grosu; BOSS XIV - 14th Belgian Organic Synthesis Symposium, July 13th-18th, 2014, Louvain-la-Neuve, Belgium; poster: "New Linear Fluorescent Antimicrobial Peptides".
-
Maria-Cristina Pascanu, Niculina D. Hadade, Ion Grosu; BOSS XIV - 14th Belgian Organic Synthesis Symposium, July 13th-18th, 2014, Louvain-la-Neuve, Belgium; poster: "Synthesis of new peptide-based cryptands".
-
M. I. Rednic, A. Terec, N. D. Hadade, I. Grosu; Cryptands and cyclophanes with phenothiazine units- oral presentation, Zilele Academice Iesene, September 24-26th 2015.
- Ion Grosu, – plenary lecture - Self-assembled Supramolecular Architectures, Innovative Host Molecules and Porous Polymers; A XXXIV-a Conferinta Nationala de Chimie, Caciulata, 04-07 October 2016.
- Ion Grosu – plenary lecture - Self-assembled Supramolecular Architectures, Innovative Host Molecules and Porous Polymers; Zilele Universitatii Al. I. Cuza, Iasi; Conferinta Facultatii de Chimie, 27-29th October 2016.
-
The yearly scientific reports containing experimental details of unpublished compounds data can be provided for project evaluation purposes only. To obtain copies of these reports please contact Prof. Dr. Ion Grosu by e-mail at igrosu (at) chem.ubbcluj.ro .