Proiect PN-II-ID-PCE-2011-3-0659:
Project timespan
Project team
Abstract
Budget
Goals
Objectives
Results
Scientific reports
Title: New metal complexes with metal-chalcogen bonds -- potential precursors for electronic materials
Project manager: Prof. Dr. Anca Silvestru
Project team:
- Conf. Dr. Monica Venter
- Conf. Dr. Richard A. Varga
- Dr. Alexandra Pop
- CSIII Dr. Alpar Pöllnitz
- Dr. Raluca Mitea
- Ana-Maria Toma
- Cristina Coza
- Eleonora Denes
- Nora Chiorean - retired
- Dorel Oltean - retired
Project timespan
2012 -- 2016
2012 -- 2016
Abstract
The synthesis based on high temperature decomposition of organometallic precursors has become one of the most successful methods of obtaining group II-VI, III-V, IV-VI or ternary colloidal nanocrystals. We propose here a research project regarding some new classes of coordination compounds which can act as precursors for new materials, either thin films or nanopowders. Thermal decomposition and CVD techniques are envisaged to obtain nanomaterials.
The main goal is to obtain new complexes with metal-chalcogen bonds, mainly with monomeric structure and good volatility, suitable as single source precursors for optoelectronic or magnetic materials. In order to avoid intermolecular associations, we decided to combine the stabilization effect of organic groups with pendant arms and donor atoms capable for intramolecular coordination with the high flexibility of a new class of dichalcogenolato organophosphorus(V) ligands. In order to obtain new metal complexes with metal-chalcogen bonds we will follow different routes, starting from metal halides. Our interests are focused on several species containing d-metals (Fe, Co, Ni, Pd, Cu, Ag, Zn, Cd) or main group metals (Sn, Sb), respectively, which can act as precursors either for nanopowders or thin films, taking in account their specific electronic, optical and magnetic properties.
Structural characterization of the new chemical species both in solution and in solid state will be performed by using different spectroscopic methods: multinuclear NMR spectroscopy (1H, 13C, 31P, 77Se, 125Te), 2D experiments and dynamic NMR, IR, RAMAN, mass spectrometry, single-crystal and powder X-ray diffraction, ESR for paramagnetic species. The obtained thin films or nanopowders will be investigated by specific methods: SEM, TEM, EDS, EXAFS.
The synthesis based on high temperature decomposition of organometallic precursors has become one of the most successful methods of obtaining group II-VI, III-V, IV-VI or ternary colloidal nanocrystals. We propose here a research project regarding some new classes of coordination compounds which can act as precursors for new materials, either thin films or nanopowders. Thermal decomposition and CVD techniques are envisaged to obtain nanomaterials.
The main goal is to obtain new complexes with metal-chalcogen bonds, mainly with monomeric structure and good volatility, suitable as single source precursors for optoelectronic or magnetic materials. In order to avoid intermolecular associations, we decided to combine the stabilization effect of organic groups with pendant arms and donor atoms capable for intramolecular coordination with the high flexibility of a new class of dichalcogenolato organophosphorus(V) ligands. In order to obtain new metal complexes with metal-chalcogen bonds we will follow different routes, starting from metal halides. Our interests are focused on several species containing d-metals (Fe, Co, Ni, Pd, Cu, Ag, Zn, Cd) or main group metals (Sn, Sb), respectively, which can act as precursors either for nanopowders or thin films, taking in account their specific electronic, optical and magnetic properties.
Structural characterization of the new chemical species both in solution and in solid state will be performed by using different spectroscopic methods: multinuclear NMR spectroscopy (1H, 13C, 31P, 77Se, 125Te), 2D experiments and dynamic NMR, IR, RAMAN, mass spectrometry, single-crystal and powder X-ray diffraction, ESR for paramagnetic species. The obtained thin films or nanopowders will be investigated by specific methods: SEM, TEM, EDS, EXAFS.
Budget
| Budget chapter (expenses) | 2012 (lei) | 2013 (lei) | 2014 (lei) | 2015 (lei) | 2016 (lei) | Total | |
|---|---|---|---|---|---|---|---|
| 1 | Salaries | 230.000 | 154.215 | 148.958 | 110.000 | 191.331 | 834.504 |
| 2 | Inventory | 167.590,96 | 18.776,29 | 15.000 | 17.408 | 70.472,54 | 287.410,09 |
| 3 | Mobility | 22.409,04 | 16.634,87 | 16.042 | 5.000 | 16.162,30 | 82.593,91 |
| 4 | Overhead | 105.000 | 47.406,54 | 45.000 | 33.102 | 69.491,46 | 300.000 |
| 5 | Total | 525.000 | 237.032,70 | 225.000 | 165.510 | 347.457,30 | 1.500.000 |
Goals
1. Design of new organochalcogen (Se, Te)-ligands, containing aromatic groups with pendant arms with donor atoms, like those depicted in Scheme 1.
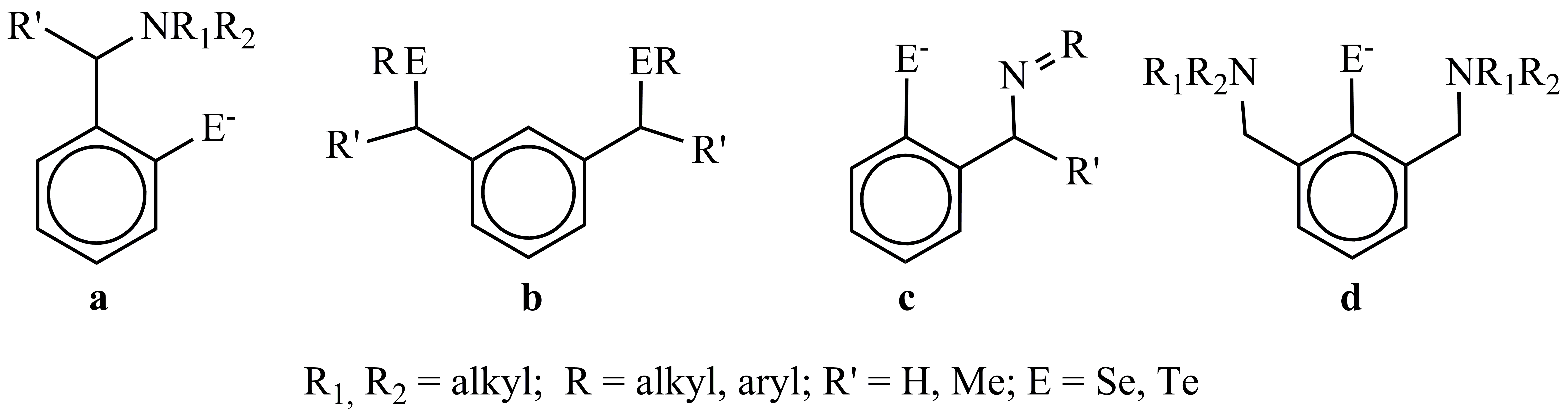
Scheme 1
2. Design of new organophosphorus ligands (L) as those depicted in Scheme 2:
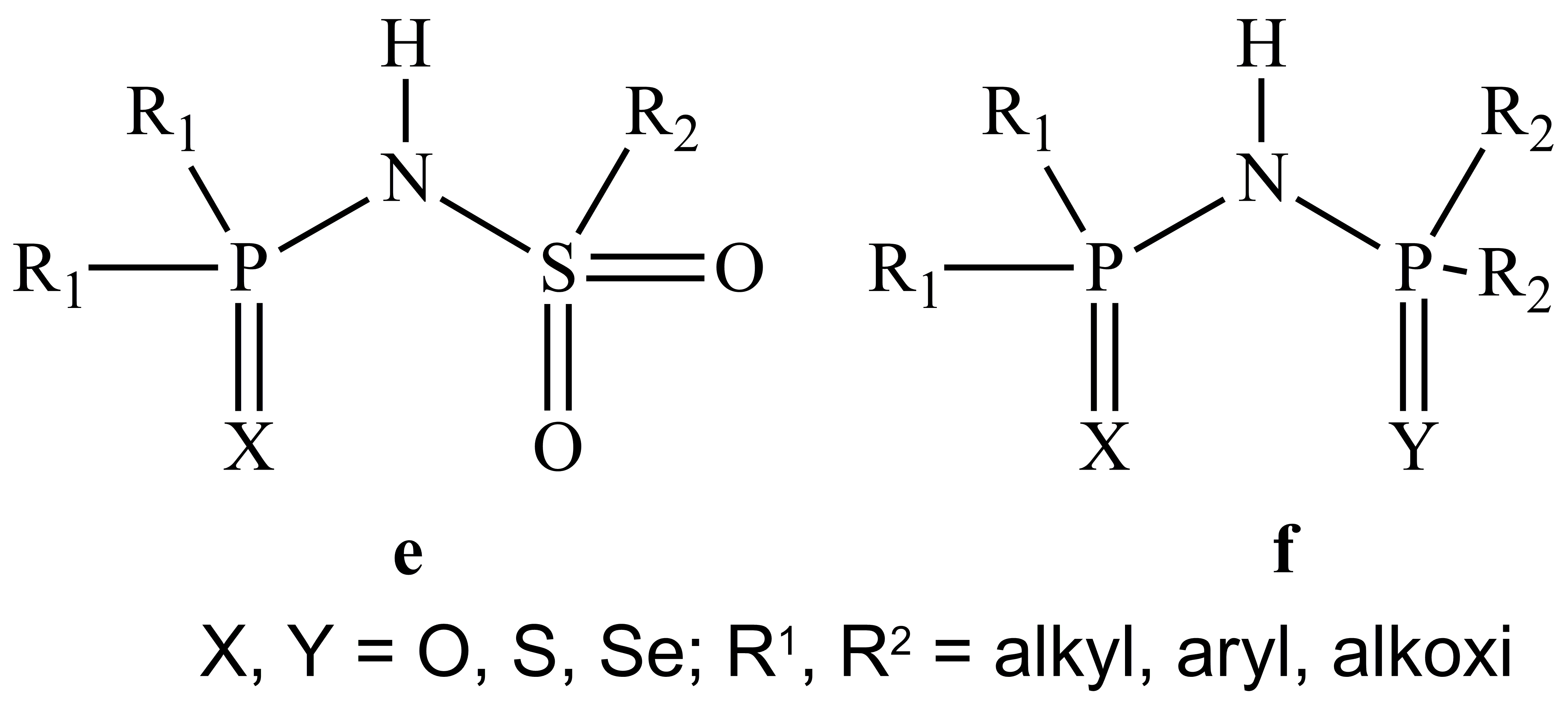
Scheme 2
3. Design of new dialcoxo and carboxilato ligands, as depicted in Scheme 3. Such ligands, containing both hard and soft donor atoms is expected to chelate metal centers and to stabilize monomeric species.
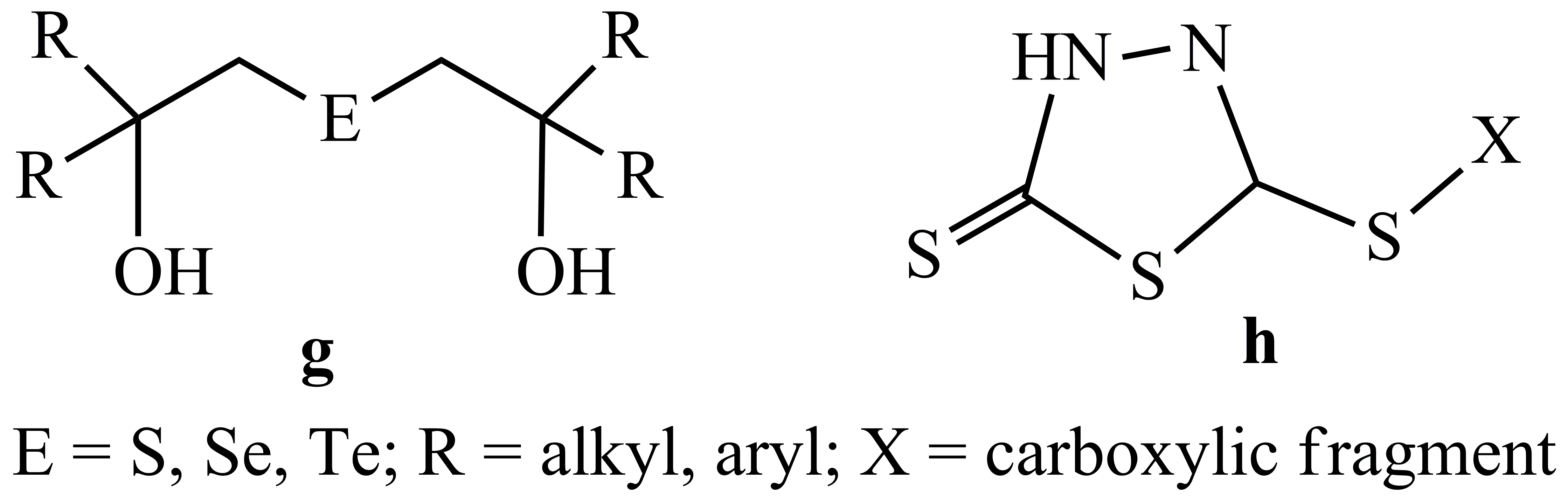
Scheme 3
4. d-metal complexes with organochalcogen ligands
In order to obtain new metal complexes with metal-chalcogen bonds in monomeric form, we will follow different routes, as depicted in Scheme 4, starting from metal halides:
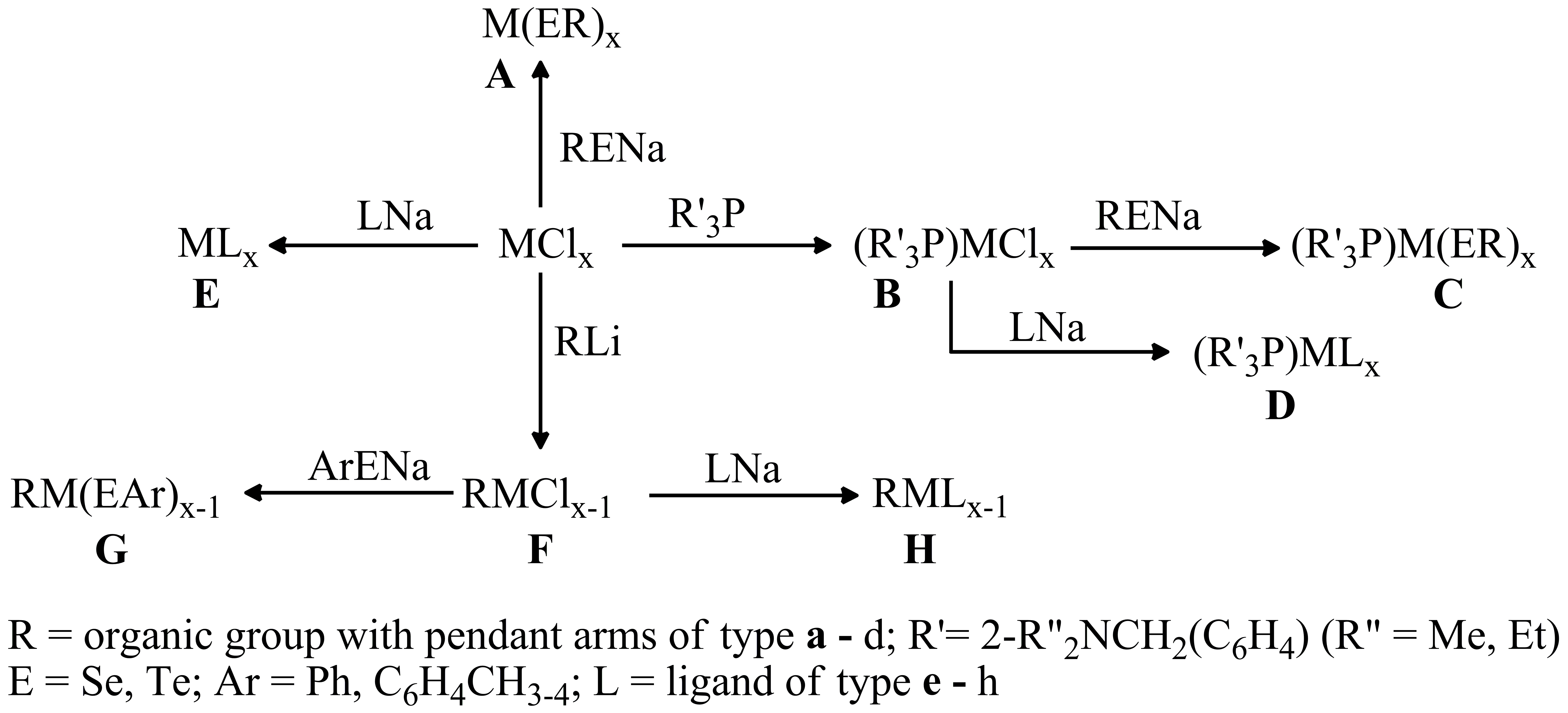
Scheme 4
Triorganophosphanes are well-known as stabilizing ligands for different metal centres (e.g. Cu, Ag, Pd, Pt, etc.). We intend to use ligands of type P[2-(R2NCH2)C6H4]xPh3-x (R = Me, Et; x = 1-3) which can prevent intermolecular associations by additional N?M intramolecular interactions.
We envisage mainly group 11 and 12 metal complexes which should be suitable as single-source precursors for CVD processes. Some group 12 species, as well as other d-metal complexes mainly with ligands of type e - h (Fe2+/3+, Co2+/3+, Ni2+, Pd2+) are envisaged also as precursors for nanopowders.
5. Main group metal complexes with organochalcogen ligandsSome main group (Sn, Sb) complexes with the same types of ligands are also envisaged.
6. Structural characterisation of the new chemical species both in solution and in solid state, using appropriate spectroscopic methods.
7. Studies concerning the volatility and the thermal behavior of the metal complexes.
8. Studies concerning the use of the new species in single source MOCVD processes will be carried out and the composition and structure of the obtained thin films will be analysed.
1. Design of new organochalcogen (Se, Te)-ligands, containing aromatic groups with pendant arms with donor atoms, like those depicted in Scheme 1.

Scheme 1
2. Design of new organophosphorus ligands (L) as those depicted in Scheme 2:
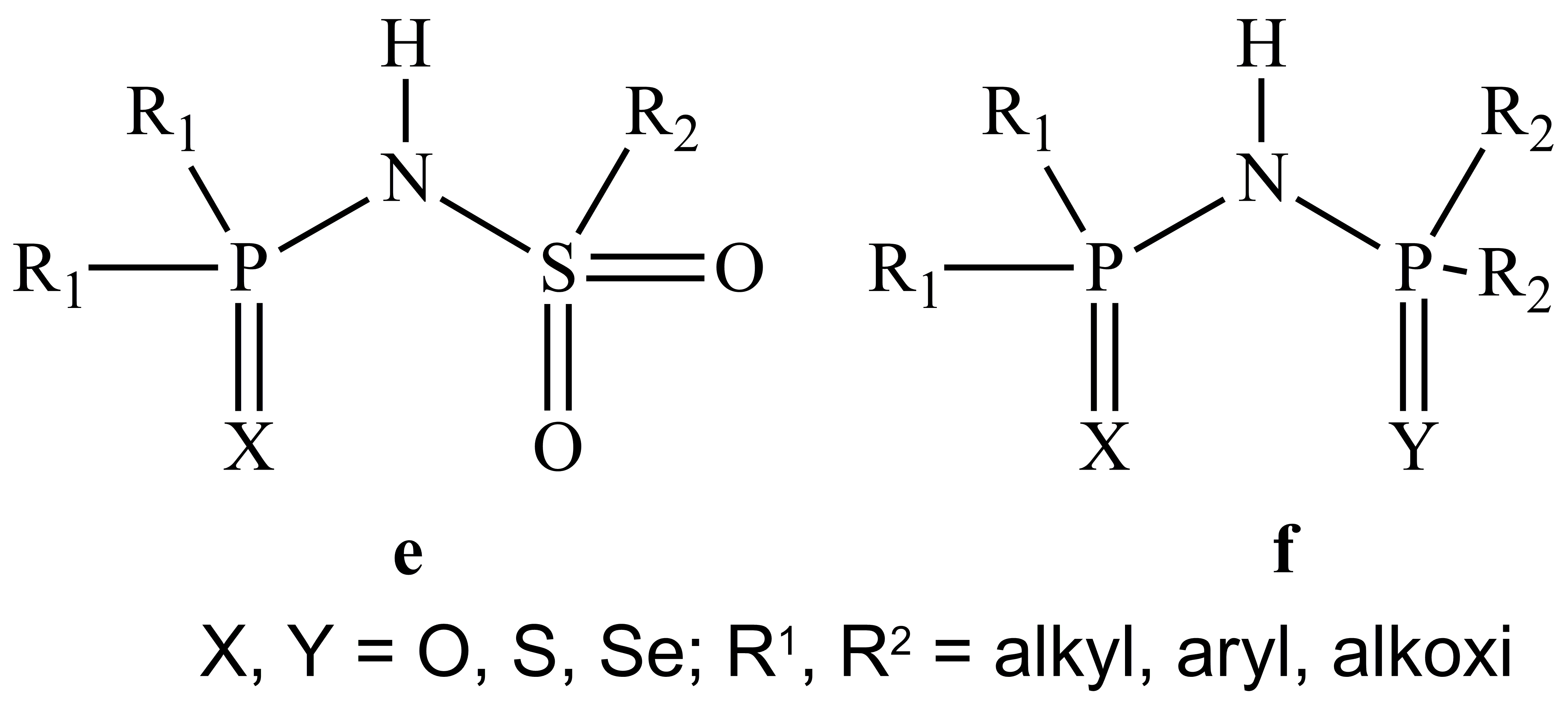
Scheme 2
3. Design of new dialcoxo and carboxilato ligands, as depicted in Scheme 3. Such ligands, containing both hard and soft donor atoms is expected to chelate metal centers and to stabilize monomeric species.
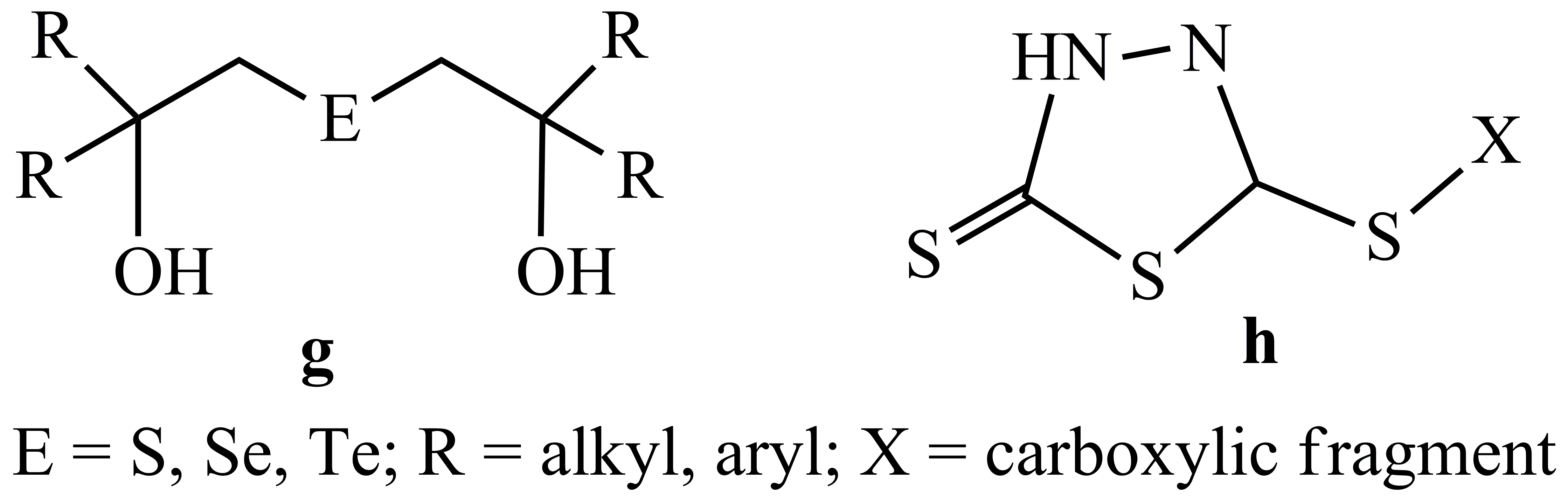
Scheme 3
4. d-metal complexes with organochalcogen ligands
In order to obtain new metal complexes with metal-chalcogen bonds in monomeric form, we will follow different routes, as depicted in Scheme 4, starting from metal halides:

Scheme 4
Triorganophosphanes are well-known as stabilizing ligands for different metal centres (e.g. Cu, Ag, Pd, Pt, etc.). We intend to use ligands of type P[2-(R2NCH2)C6H4]xPh3-x (R = Me, Et; x = 1-3) which can prevent intermolecular associations by additional N?M intramolecular interactions.
We envisage mainly group 11 and 12 metal complexes which should be suitable as single-source precursors for CVD processes. Some group 12 species, as well as other d-metal complexes mainly with ligands of type e - h (Fe2+/3+, Co2+/3+, Ni2+, Pd2+) are envisaged also as precursors for nanopowders.
5. Main group metal complexes with organochalcogen ligandsSome main group (Sn, Sb) complexes with the same types of ligands are also envisaged.
6. Structural characterisation of the new chemical species both in solution and in solid state, using appropriate spectroscopic methods.
7. Studies concerning the volatility and the thermal behavior of the metal complexes.
8. Studies concerning the use of the new species in single source MOCVD processes will be carried out and the composition and structure of the obtained thin films will be analysed.
Objectives accomplished in 2012 - 2013
- Design of new organophosphorus ligands with XPNSO skeleton. Synthesis and structural characterization.
- Design of new triorganophosphanes. Synthesis and structural characterization.
- Design of new organochalcogen (Se, Te)-ligands and their group 12 metal complexes; Synthesis, structural, characterization, thermal behavior.
- Metal complexes with organophosphorus ligands. Synthesis and structural characterization.
- Design of new organochalcogen (Se, Te)-ligands and their group 11 metal complexes.
- Organometallic group 12 and main group (Sb, Sn) metal complexes with organochalcogen (Se, Te)-ligands; Synthesis and structural characterization, thermal behavior.
- Design of new dialcoxo and carboxilato ligands and their d metal complexes; Synthesis and structural characterization, thermal behavior.
- Design of new d-metal complexes with organophosphorus ligands with XPNSO and XPNPO skeleton. Synthesis and structural characterization.
Objectives accomplished in 2014
- Design of new organo-chalcogen ligands.
- Synthesis of new compounds derived from the hypervalent diorganodiselenides [2,6-(R2NCH2)C6H3]2Se2 (R = Me, R2 = O(CH2CH2)2, MeN(CH2CH2)2.
- New organometallic compounds of main group metals (Sb, Bi or Sn) with organo-chalcogen ligands.
- New complexes of d - metals with organo-chalcogen-, alkoxo or carboxilato ligands.
- Thermal behavior of the new precursors.
- Final characterization of several compounds obtained in 2012 and 2013.
Objectives accomplished in 2015
- Studies regarding the chemical reactivity of several new organochalcogen ligands
- Synthesis and structural characterization of new organometallic compounds of main group metals (Sb, Bi or Sn) with organochalcogen (Se, Te) ligands
- Synthesis and structural characterization of new coordination compounds of d-block metals with organochalcogen (Se, Te) ligands
- Thermal behavior of the new species
- Final characterization of several compounds obtained in 2012 - 2014.
Results
Articles published in 2012-2016
1. Organophosphorus ligands with XPNSO skeleton (X = O, S) and their Pd(II) complexes. Crystal and molecular structure of [(EtO)2P(S)NSO2R]H [R = Me, C6H4CH3-4, C6H4Cl-4 and [Pd{(SPPh2)(O2SC6H4Cl-4)N}2].
D. Oltean, A. Pöllnitz, A. Silvestru, Polyhedron 53 (2013) 67-75. DOI: 10.1016/j.poly.2013.01.023.
2. Cobalt(II) complexes with hypervalent triarylphosphanes.
R. Mitea, A. Covaci, C. Silvestru, A. Silvestru, Rev. Roum. Chim. 58 (2013) 265-273.
3. Organoantimony(III) and -bismuth(III) hypervalent pseudohalides. An experimental and theoretical study.
A. Toma, C. I. Rat, A. Silvestru, T. Rüffer, H. Lang, M. Mehring, J. Organomet Chem. 745-746 (2013) 71-79. DOI: 10.1016/j.jorganchem.2013.06.044.
4. New diorganoselenium(II) compounds and their behavior towards late transition metals.
A. Pop, D. Rosca, R. Mitea, A. Silvestru, Inorg. Chim. Acta 405 (2013) 235-242. DOI: 10.1016/j.ica.2013.06.003.
5. New copper(I) complexes with organophosphorus ligands with XPNSO skeleton.
C. Strady, A. Stegarescu, C. Silvestru, A. Silvestru, Studia Universitatis Babes-Bolyai, Seria Chemia, 4 (2013) LVIII 243-251.
6. Organoselenium(II) halides containing the pincer ligand 2,6-(Me2NCH2)2C6H3 - an experimental and theoretical study.
A. Pop, A. Silvestru, E. J. Juarez-Perez, M. Arca, V. Lippolis, C. Silvestru, Dalton Trans. 43 (2014) 2221-2233. DOI: 10.1039/c3dt52886c.
7. Copper(I) complexes with the triarylphosphanes PPhn(C6H4CH2NMe2-2)3-n (n = 0-2) and PPh2[C6H4CH2N(CH2CH2)2O-2]. Synthesis and structural characterization.
A. Covaci, R. Mitea, I. Hosu, A. Silvestru, Polyhedron 72 (2014) 157-163. DOI: 10.1016/j.poly.2014.01.035.
8. Diorganochalcogen(II) ligands of type [R2C(OH)CH2](2-Me2NCH2C6H4)E (E = S, Se, Te; R = Me, Ph), and their silver(I) complexes.
A. Pop, R. Mitea, A. Silvestru, J. Organomet. Chem. 768 (2014) 121-127. DOI: 10.1016/j.jorganchem.2014.06.007.
9. On the coordination chemistry of organochalcogenolates RNMe2^E- and RNMe2^E^O- (E = S, Se) onto lead(II) and lighter divalent tetrel elements.
A. Pop, L. Wang, V. Dorcet, T. Roisnel, J.-F. Carpentier, A. Silvestru, Y. Sarazin, Dalton Trans. 43 (2014) 16459 - 16474. DOI: 10.1039/c4dt02252a.
10. Spectroscopic and thermal studies on the iron(III) mercapto-thiadiazol-thiosuccinate precursor for iron(III) oxides.
M. M. Venter, V. N. Bercean, F. Goga, M. Nasui, Rev. Roum. Chim. 59 (2014) 989-996. DOI: n.a.
11. New tripheniltelluronium salts of organophosphorus ligands.
N. Chiorean, D. Margineanu, A. Silvestru, Rev. Roum. Chim. 59 (2014) 953-958. DOI: n.a.
12. New diorganodichalcogenides and cyclization reactions leading to 3-benzilamino- benzo[b]chalcogenophens.
A. Pollnitz, A. Silvestru, Tetrahedron 71 (2015) 2914-2921. DOI: 10.1016/j.tet.2015.03.058
13. Group 12 metal complexes with triarylphosphanes of type PRxPh3-x [R = 2-(Me2NCH2)C6H4, 2-{O(CH2CH2)2NCH2}C6H4, x = 1 - 3].
A. Covaci, I. Covaci, A. Silvestru, Dalton Trans. under evaluation.
14. Cobalt(II) complexes of organophosphorus ligands with XPNSO skeleton (X = O, S). Solid state structure and solution behavior.
E. Denes, A. Pollnitz, F. Cziple, M. Vlassa, A. Silvestru, Inorg. Chim. Acta 444 (2016) 23-28. DOI: 10.1016/j.ica.2016.01.018.
15. Heterocyclic bismuth(III) compounds with transannular S→Bi interactions. An experimental and theoretical approach.
A. Toma, C. I. Rat, A. Silvestru, T. Rueffer, H. Lang, M. Mehring, J. Organomet. Chem. 806 (2016) 5-11. DOI: 10.1016/j.jorganchem.2016.01.019.
16. Bis(2-phenoxyphenyl)dichalcogenides and their chemical reactivity.
A. Toma, A. Nicoara, A. Silvestru, T. Rueffer, H. Lang , M. Mehring, J. Organomet. Chem. 810 (2016) 33-39. DOI: 10.1016/j.jorganchem.2016.03.002.
17. Silver(I) complexes of a new multidentate macrocyclic ligand with N/S/Se donor atoms.
R. A. Popa, A. Silvestru, A. Pop, Polyhedron 110 (2016) 197-202. DOI: 10.1016/j.poly.2016.02.045.
18. Organoselenium compounds with (N,C,N) pincer ligands. Structure and chemical behaviour.
A. Pop, A. Silvestru, J. Organomet. Chem. under evaluation.
19. New hypervalent organoselenium compounds with O→Se intramolecular interactions.
R. A. Popa, A. Pop, C. Silvestru, A. Silvestru, Rev. Roum. Chim. 61 (2016) 495-501.
20. New diorganochalcogen compounds based on the 2-(Me2NCH2)C6H4 group.
B. Danciu, R. A. Popa, A. Pop, V. Zaharia, C. Silvestru and A. Silvestru, Studia Universitatis Babes-Bolyai, Seria Chemia, LXI (2016) 19-28.
Conferences attended in 2012-2016
Articles published in 2012-2016
1. Organophosphorus ligands with XPNSO skeleton (X = O, S) and their Pd(II) complexes. Crystal and molecular structure of [(EtO)2P(S)NSO2R]H [R = Me, C6H4CH3-4, C6H4Cl-4 and [Pd{(SPPh2)(O2SC6H4Cl-4)N}2].
D. Oltean, A. Pöllnitz, A. Silvestru, Polyhedron 53 (2013) 67-75. DOI: 10.1016/j.poly.2013.01.023.
Several organophosphorus acids of type [{XP(OEt2}(O2SR)]NH [X = O, R = Me (1), Ph (2), C6H4CH3-4 (3); X = S, R = C6H4Cl-4 (4)] were obtained by a method based on the reaction between (EtO)2P(X)NHLi and the corresponding organosulphonyl chloride. They were subsequently deprotonated with KOBut. Salt metathesis reactions between PdCl2 and the corresponding potassium salts K[{XP(OEt)2}(O2SR)N] [X = O, R = Me (5), Ph (6), C6H4CH3-4 (7); X = S, R = C6H4Cl-4 (8)] in a 1:2 molar ratio resulted in palladium(II) complexes of type Pd[{XP(OEt)2}(O2SR)N]2 [X = O, R = Me (9), Ph (10), C6H4CH3-4 (11); X = S, R = C6H4Cl-4 (12)]. In a similar way was obtained the palladium(II) complex Pd[(SPPh2)(O2SPh)N]2 (13), using the appropriate reagents. All compounds were characterized by multinuclear NMR (1H, 13C, 31P) and infrared spectroscopy. The potassium salts and the palladium complexes were investigated also by mass spectrometry. Single-crystal X-ray diffraction studies revealed a layered supramolecular assembly in case of acid 1, a monomeric structure for compound 4, while in case of compound 2 dimeric associations are formed in the solid state by NH···OP hydrogen bonding. A trans-S,N- coordination pattern of the organophosphorus ligand was found in the palladium complex 12. Moreover, short Cl···H contacts result in a polymeric [Pd[{SP(OEt)2}(O2SC6H4Cl-4)N]2]n chain.
2. Cobalt(II) complexes with hypervalent triarylphosphanes.
R. Mitea, A. Covaci, C. Silvestru, A. Silvestru, Rev. Roum. Chim. 58 (2013) 265-273.
Reactions between triarylphosphanes of type P(C6H4CH2NMe2-2)xPh3-x [x = 1 (1), 2 (2), 3 (3)] and CoCl2·6H2O in a 1:1 molar ratio resulted in cobalt(II) complexes of type [CoCl2{P(C6H4CH2NMe2-2)XPh3-x}] [x = 1 (4), 2 (5), 3 (6)]. The new species were isolated as blue solids and they were investigated by 1H and 31P NMR, mass spectrometry and electronic spectroscopy. X-ray diffraction studies revealed a monomeric structure in case of the starting phosphane P(C6H4CH2NMe2-2)2Ph (2), in which one 2-(Me2NCH2)C6H4 pendant arm is involved in an intramolecular N→P interaction. The oxidized species [CoCl2{OP(C6H4CH2NMe2-2)xPh3-x}] [x = 2 (5a), 3 (6a)] were also isolated as blue solids. A similar coordination pattern as in case of the related [ZnCl2{OP(C6H4CH2NMe2-2)2Ph}] (7) might be proposed for such oxidized species.
3. Organoantimony(III) and -bismuth(III) hypervalent pseudohalides. An experimental and theoretical study.
A. Toma, C. I. Rat, A. Silvestru, T. Rüffer, H. Lang, M. Mehring, J. Organomet Chem. 745-746 (2013) 71-79. DOI: 10.1016/j.jorganchem.2013.06.044.
The reaction between the organopnicogen(III) chlorides R2MCl and RMCl2 [M = Sb, Bi; R = 2-(Me2NCH2)C6H4] and alkali metal pseudohalides in a 1:1 and a 1:2 molar ratio, respectively, resulted in the formation of hypervalent species of type R2MX (M = Sb, X = NCO (1), NCS (2) and SeCN (3), M = Bi, X = NCO (4) and SeCN (5), and RMX2 (M = Sb, X = NCO (6) and NCS (7), M = Bi, X = NCO (8), NCS (9) and SeCN (10). The new species were characterized by multinuclear NMR (1H, 13C, 77Se where appropriate), IR spectroscopy and mass spectrometry. The molecular structures of compounds 2-5 and 7 were determined by single-crystal X-ray diffraction. In all five compounds the nitrogen atoms in the pendant arms are involved in intramolecular coordination to metal, thus resulting in a distorted square pyramidal coordination geometry (12-M-5 hypervalent species) in case of 2-5 and a distorted trigonal pyramidal coordination geometry in case of 7(10-Sb-4 hypervalent species). The intramolecular N→M coordination determines a chiral pattern of the investigated compounds. Bi···Phcentroid intermolecular interactions led to dimers in case of compound 4. In all species weak CH···Phcentroid intermolecular contacts led to polymeric associations, e.g. polymeric chains in case of compounds 2, 3, 5 and 7 and a 2D layer structure built by chains of dimers in case of compound 4. DFT calculations and FTIR data allowed us to assign the coordination pattern of the pseudohalide ligand in the other species.
4. New diorganoselenium(II) compounds and their behavior towards late transition metals.
A. Pop, D. Rosca, R. Mitea, A. Silvestru, Inorg. Chim. Acta 405 (2013) 235-242. DOI: 10.1016/j.ica.2013.06.003.
Hypervalent diorganoselenium(II) compounds of type 1-{2-[(2-(3,5-dimethyl-4,5-dihydro-1H-pyrazol-1-yl)ethyl)selanyl]phenyl}-N,N-dialkylmethanamine, [2-(R2NCH2)C6H4][(3,5-dmpz)CH2CH2]Se, [R = Me (1), Et (2), dmpz = dimethylpyrazole] were obtained by reacting 1-(2-bromoethyl)-3,5-dimethyl-1H-pyrazole [(3,5-dmpz)CH2CH2Br] with alkali metal aryl selenolates of type [2-(R2NCH2)C6H4]SeM (R = Me, M = Na; R = Et, M = Li) in a 1:1 molar ratio. The coordination ability of species 1, as well as that of bis[2-(3,5-dimethyl-1H-pyrazol-1-yl)ethyl]selane, [(3,5-dmpz)CH2CH2]2Se (3) towards several late transition metals (Pd, Au, Ag) was investigated by reacting the neutral ligands 1 and 3 with PdCl2, AuCl(tht) and AgOTf in a 1:1 molar ratio. The neutral ligands 1-3, as well as the gold(I), silver(I) and palladium(II) adducts [2-(Me2NCH2)C6H4][(3,5-dmpz)CH2CH2)]SeAuCl (4), [(3,5-dmpz)CH2CH2)]2SeAuCl (5), [2-(Me2NCH2)C6H4][(3,5-dmpz)CH2CH2)]SeAgOTf (6), [(3,5-dmpz)CH2CH2)]2SeAgOTf (7) and [(3,5-dmpz)CH2CH2)]2SePdCl2 (8) were characterized by NMR spectroscopy (1H, 13C, 77Se) and mass spectrometry. The reaction between 1 and PdCl2 in a 1:1 molar ratio resulted in the formation of a complex reaction mixture of which the species [2-(Me2NCH2)C6H4SePdCl]2 (9) and 1-(2-chloroethyl)-3,5-dimethyl-1H-pyrazole [(3,5-dmpz)CH2CH2Cl] were unambigously identified. Single crystal X-ray diffraction studies revealed a monomeric structure with a distorted T-shaped coordination geometry of type (C,N)SeC for the neutral ligand 1 and supramolecular associations based on dimeric units in case of species 9 and 9·0.5CH2Cl2·0.5THF.
5. New copper(I) complexes with organophosphorus ligands with XPNSO skeleton.
C. Strady, A. Stegarescu, C. Silvestru, A. Silvestru, Studia Universitatis Babes-Bolyai, Seria Chemia, 4 (2013) LVIII 243-251.
Salt metathesis reactions between K[{SP(OEt)2}(O2SC6H4Cl-4)N] and the copper(I) species CuCl and (Ph3P)2CuNO3, respectively, resulted in the new complexes [Cu({SP(OEt)2}(O2SC6H4Cl-4)N)] (1) and [Cu(PPh3)2({SP(OEt)2}(O2SC6H4Cl-4)N)] (2). The two compounds were characterized by multinuclear NMR (1H, 13C, 31P). Single-crystal X-ray diffraction studies revealed a monomeric structure with a bidentate, monometallic biconnective behavior of the organophosphorus(V) ligand in case of compound 2. For compound 1 the NMR spectra suggest intermolecular associations in solution.
6. Organoselenium(II) halides containing the pincer ligand 2,6-(Me2NCH2)2C6H3 - an experimental and theoretical study.
A. Pop, A. Silvestru, E. J. Juarez-Perez, M. Arca, V. Lippolis, C. Silvestru, Dalton Trans. 43 (2014) 2221-2233. DOI: 10.1039/c3dt52886c.
New organoselenium(II) halides of type RSeX [R = 2,6-(Me2NCH2)2C6H3] were prepared by cleavage of the Se−Se bond in R2Se2 with SO2Cl2 to give RSeCl followed by halogen exchange with NaBr or KI. The reaction between RSeCl and R'2MCln [R' = Ph, 2-(Me2NCH2)C6H4; M = Sb, Bi; n = 1 or 3) resulted in new ionic species of type [RSe]+[R'2MCln]− (n = 2 or 4). All new compounds were investigated in solution by multinuclear NMR spectroscopy (1H, 13C, 77Se, 2D and VT experiments) and mass spectrometry. The molecular structures of [{2,6-(Me2NCH2)2C6H3}Se]+Cl−·2H2O and [{2,6-(Me2NCH2)2C6H3}Se]+[Ph2SbCl4]−, respectively, were established by single-crystal X-ray diffraction, pointing out the ionic nature of these compounds, with both nitrogen atoms strongly coordinated trans each other to the selenium atom of the cation. DFT calculations were performed in order to elucidate the bond nature and vibrational spectroscopic features of this class of organoselenium(II) compounds.
7. Copper(I) complexes with the triarylphosphanes PPhn(C6H4CH2NMe2-2)3-n (n = 0-2) and PPh2[C6H4CH2N(CH2CH2)2O-2]. Synthesis and structural characterization.
A. Covaci, R. Mitea, I. Hosu, A. Silvestru, Polyhedron 72 (2014) 157-163. DOI: 10.1016/j.poly.2014.01.035.
Several copper(I) complexes of type [CuXPPhn(C6H4CH2NMe2-2)3-n] [X = Cl, n = 1 (2), 2 (3); X = I, n = 0 (4), 1 (5), 2 (6)] were prepared by reacting CuX with the appropriate triarylphosphane in a 1:1 molar ratio. The ionic species [Cu(MeCN)2PPh2{C6H4CH2N(CH2CH2)2O-2}]+PF6- (7) was obtained in the reaction between [Cu(MeCN)4]PF6 and PPh2{C6H4CH2N(CH2CH2)2O-2} (1). All compounds were characterized by NMR spectroscopy (1H and 31P) and mass spectrometry. For compound 7 the 19F NMR spectrum was also recorded. Single-crystal X-ray diffraction studies put in evidence a P,N monometallic biconnectiv coordination behavior of the ligand in compounds 3, 6·0.5CH2Cl2 and 7·CH2Cl2, while in compound 4 a P,N,N monometallic triconnectiv behavior was observed. In species 2 the unit cell contains two independent molecules; in one of them the PN2 ligand behaves P,N,N-monometallic triconnective, while in the other as a P,N-monometallic biconnective moiety. The N→Cu intramolecular coordination induces planar chirality to the metal center and, as a consequence, the crystal contains both R and S isomers.
8. Diorganochalcogen(II) ligands of type [R2C(OH)CH2](2-Me2NCH2C6H4)E (E = S, Se, Te; R = Me, Ph), and their silver(I) complexes.
A. Pop, R. Mitea, A. Silvestru, J. Organomet. Chem. 768 (2014) 121-127. DOI: 10.1016/j.jorganchem.2014.06.007.
Ligands of type [R2C(OH)CH2](2-Me2NCH2C6H4)E [R = Me, E = S (1), Se (2), Te (3); R = Ph, E = S (4), Se (5)] were obtained in the reactions of (2-Me2NCH2C6H4)ELi and the corresponding R2C(OH)CH2Cl (R = Me, Ph) reagent. The reactions between the ligands 1 - 3 and 5 with AgOTf (OTf = OSO2CF3), in a 1:1 molar ratio, resulted in the silver(I) complexes [AgOTf{E[CH2C(OH)R2](C6H4CH2NMe2-2)}] [R = Me, E = S (6), Se (7), Te (8); R = Ph, E = Se (9)]. The new compounds 1 - 9 were investigated by solution NMR spectroscopy (1H, 19F, 77Se, 125Te, as appropriate). The ligands 1 - 5 were investigated also by 13C NMR. The IR spectra and the molar conductivity of the silver(I) complexes suggested a covalent nature of the Ag-OTf bond, while their ESI+ MS spectra suggest the formation of dimeric species. Single crystal X-ray diffraction studies revealed a monomeric structure with intramolecular N···H-O hydrogen bonding for compound 5, while for the complex 6 dimeric associations formed by bridging ligands were found.
9. On the coordination chemistry of organochalcogenolates RNMe2^E- and RNMe2^E^O- (E = S, Se) onto lead(II) and lighter divalent tetrel elements.
A. Pop, L. Wang, V. Dorcet, T. Roisnel, J.-F. Carpentier, A. Silvestru, Y. Sarazin, Dalton Trans. 43 (2014) 16459 - 16474. DOI: 10.1039/c4dt02252a.
Several families of heteroleptic tetrelenes of general formulae M(E^RNMe2)[N(SiMe3)2] and M(O^E^RNMe2)[N(SiMe3)2] (where E = S, Se; M = Ge, Sn, Pb; RNMe2 = 2-(Me2NCH2)C6H4] supported by organochalcogenolato ligands have been prepared and fully characterised. The coordination chemistry of these ligands containing both hard (N, O) and soft (S, Se) atoms onto metals of varying size, polarisability, electropositivity and electrostatic surface potential has been explored. In the molecular solid-state, the complexes M(E^RNMe2)[N(SiMe3)2] are monomeric, although an occurrence of weak Pb···Se intermolecular interactions yielding a bimolecular species has been identified in the case of the plumbylene Pb[SeC6H4(CH2NMe2)-2][N(SiMe3)2]. On the other hand, all complexes M(O^E^RNMe2)[N(SiMe3)2] form centro-symmetric bimetallic dimers with O-bridging atoms. Multinuclear (29Si, 77Se, 119Sn, 207Pb) NMR spectroscopy and crystallographic studies reveal that the metal preferably remains 3-coordinated in all these heteroleptic complexes with absence of coordination of N and S/Se atoms, unless severe depletion of electronic density onto the metal is enforced. Coordination of these heteroelements can thus be achieved either through replacement of a-CH3 substituents (as in the ligand 2-(Me2NCH2)C6H4SeCH2C(Me)2O-) by electron-withdrawing a-CF3 moieties (as in the ligand 2-(Me2NCH2)C6H4SeCH2C(CF3)2O-), or else with recourse to the use of a cationizing agent leading to the formation of the ion pair [{2-(Me2NCH2)C6H4SeCH2C(Me)2O}Pb]+·[H2N{B(C6F5)3}2]- where the cationic metal complex is associated to a weakly-coordinating anion. The data collated herein provide compelling evidence that the coordination chemistry of divalent tetrel elements with ligands featuring both hard and soft donors cannot be reliably anticipated by sole use of general concepts such as the HSAB theory. The related metal complexes containing the rigid 8-(NMe2)naphthalen-1-yl group are also discussed.
10. Spectroscopic and thermal studies on the iron(III) mercapto-thiadiazol-thiosuccinate precursor for iron(III) oxides.
M. M. Venter, V. N. Bercean, F. Goga, M. Nasui, Rev. Roum. Chim. 59 (2014) 989-996. DOI: n.a.
Reaction of the new (3H-2-thioxo-1,3,4-thiadiazol-5-yl)-thiosuccinic acid (H3L) with FeCl3·6H2O and Et3N in aqueous medium produced a new iron(III) complex [FeL]·1.5H2O, regardless the molar ratio of the reagents. The empirical formula of the product is supported by elemental analysis. Both acid and complex are characterized by vibrational spectroscopy (FT-IR, FT-Raman). The spectra are consistent with the complete deprotonation of the acid and the formation of the product. The TGA/DTA/DTG analysis of [FeL]·1.5H2O indicates the thermolysis of the precursor in four major steps, to produce Α-Fe2O3. Each solid residue is monitored by FT-IR spectroscopy. The identity of the final thermolysis product is confirmed by powder X-ray diffraction.
11. New tripheniltelluronium salts of organophosphorus ligands.
N. Chiorean, D. Margineanu, A. Silvestru, Rev. Roum. Chim. 59 (2014) 953-958. DOI: n.a.
New triorganotellurium(IV) compounds of type [Ph3Te][(R2PS)(R'SO2)N], [R = Ph, R' = Me (1), R = OEt; R' = 4-ClC6H4 (2)], as well as [Ph3Te][O2PPh2] (3) were obtained by reacting Ph3TeCl with the potassium salt of the appropriate organophosphorus acid in an 1 : 1 molar ratio. The new species were characterized by multinuclear NMR (1H, 13C, 31P and 125Te) and mass spectrometry. The molar conductivity in DMSO solution suggested their behaviour as 1 : 1 electrolytes. The single-crystal X-ray diffraction structure of 3·Ph2PO2H revealed strong Te···O interactions between the triphenyltelluronium cation and the Ph2PO2– anion and the free acid, respectively.
12. New diorganodichalcogenides and cyclization reactions leading to 3-benzilamino- benzo[b]chalcogenophens.
A. Pollnitz, A. Silvestru, Tetrahedron 71 (2015) 2914-2921. DOI: 10.1016/j.tet.2015.03.058
Bis(2-acetylphenyl)dichalcogenides of type [2-(O=CMe)C6H4]2E2 (E = S, Se, Te) were prepared by the hydrolysis of [2-{(OCH2)2C(CH3)}C6H4]2E2. In their reaction with benzylamine these species yield an airsensitive condensation product, which undergoes a fast, unexpected oxidative intramolecular cyclization. This process proved to be a very convenient way to prepare 3-amino substituted benzo[b]chalcogenophenes. By contrast, the reaction between [2-(O=CMe)C6H4]TeCl and benzylamine resulted in the condensation product [2-(C6H4CH2N=CMe)C6H4]TeCl.
13. Group 12 metal complexes with triarylphosphanes of type PRxPh3-x [R = 2-(Me2NCH2)C6H4, 2-{O(CH2CH2)2NCH2}C6H4, x = 1 - 3].
A. Covaci, I. Covaci, A. Silvestru, Dalton Trans. under evaluation.
Group 12 metal complexes of type [MCl2(PRxPh3-x)] [R = C6H4(Me2NCH2)-2, M = Zn, x = 3 (2), 2 (3), 1 (4), M = Cd, x = 3 (5), 2 (6), 1 (7), R = 2-{O(CH2CH2)2NCH2}C6H4, x = 1 (8)] were prepared by complexation reactions between the metal dichloride MCl2 (M = Zn, Cd) with the corresponding hypervalent triarylphosphane in a 1:1 molar ratio. The triarylphosphane PPh2[C6H4{CH2N(CH2CH2)2O}-2] (1) was obtained by reacting the lithiated derivative Li[C6H4{CH2N(CH2CH2)2O}-2] and Ph2PCl, in a 1:1 molar ratio. The solution behaviour of the triarylphosphane 1 and the complexes 2 - 8 was investigated by 1H and 31P NMR. The molecular structures of 1, 4, 6, 7·CH2Cl2 and 8 were determined by single-crystal X-ray diffraction. In all complexes the phosphane ligand acts as a monometallic biconnective moiety, coordinated both by phosphorus and nitrogen to the metal centre. In complex 7·CH2Cl2 dimeric units are formed through Cd···Cl secondary interactions. Excepting the crystal structure of 6 which consists of discrete monomeric units, in all other complexes supramolecular assemblies based on Cl···H contacts can be considered.
14. Cobalt(II) complexes of organophosphorus ligands with XPNSO skeleton (X = O, S). Solid state structure and solution behavior.
E. Denes, A. Pollnitz, F. Cziple, M. Vlassa, A. Silvestru, Inorg. Chim. Acta 444 (2016) 23-28. DOI: 10.1016/j.ica.2016.01.018.
Cobalt(II) complexes of type Co[(XPR2)(O2SR)N]2 [X = S; R = Ph; R' = Me (1), Ph (2), C6H4CH3-4 (3); X = O; R = Ph, R' = Ph (4), R = OEt, R' = C6H4CH3-4 (5)] were prepared by salt metathesis reactions between CoCl2·6H2O and the potassium salt of the appropriate organophosphorus acid in a 1:2 molar ratio. All compounds were characterized by UV-Vis spectroscopy in CH2Cl2 solutions, mass spectrometry, infrared spectroscopy and room temperature magnetic susceptibility. For compounds 1 and 5 electronic spectra were recorded in dimethylformamide (dmfa) as well. While the electronic spectra suggest a monomeric structure in CH2Cl2 solutions with a tetrahedral environment about cobalt(II), for compound 3 in solid state a dinuclear structure was determined by single-crystal X-ray diffraction, with two bridging S,O,O-bimetallic triconective and two S,O-monometallic biconective [(SPPh2)(O2SC6H4CH3-4)N]- ligand units. From a CH2Cl2 solution of 1 crystals of the oxidation product Co(OH)[(SPPh2)(O2SMe)N]2 (6) were isolated after standing for several weeks in open atmosphere. Compound 4 in dimethylformamide decomposed and forms the octahedral species Co[(O2PPh2)(HO2PPh2)(dmfa)]2 (7) (dmfa = dimethylformamide). The redox behavior of 1 was investigated by cyclic voltammetry in CH2Cl2 solution.
15. Heterocyclic bismuth(III) compounds with transannular S→Bi interactions. An experimental and theoretical approach.
A. Toma, C. I. Rat, A. Silvestru, T. Rueffer, H. Lang, M. Mehring, J. Organomet. Chem. 806 (2016) 5-11. DOI: 10.1016/j.jorganchem.2016.01.019.
Several new diorganobismuth(III) compounds based on a butterfly-like tetrahydro-dibenzo[c,f][1,5]thiabismocine heterocyclic framework were prepared and structurally characterized. The reaction between the dilithio derivative of bis(2-bromobenzyl)sulfane with BiBr3 in a 1:1 molar ratio resulted in the formation of [(C6H4CH2)2S]BiBr (1). Further exchange reactions of 1 with KI, AgNO3 and AgOSO2CF3, respectively, afforded the hypervalent species [(C6H4CH2)2S]BiX [X = I (2), ONO2 (3) and OSO2CF3 (4)]. The new species were characterized by 1H and 13C NMR, FT IR spectroscopy and mass spectrometry. The crystal and molecular structures of compounds 1 - 4 were determined by single-crystal X-ray diffraction. In all compounds the sulfur atom is strongly intramolecularly coordinated to bismuth, thus resulting in hypervalent species 10-Bi-4 (for 1 and 2) and 12-Bi-5 (for 3 and 4). Intermolecular interactions (X···Hmethylene in 1 and 2, Bi···Cg in 3 and Bi···O in 4) led to polymeric chains in the crystals. DFT calculations were carried out on [(C6H4CH2)2S]BiCl and 1 - 4 in order to better understand the S-Bi intramolecular coordination.
16. Bis(2-phenoxyphenyl)dichalcogenides and their chemical reactivity.
A. Toma, A. Nicoara, A. Silvestru, T. Rueffer, H. Lang , M. Mehring, J. Organomet. Chem. 810 (2016) 33-39. DOI: 10.1016/j.jorganchem.2016.03.002.
Diorganodichalcogenides of type [(C6H5)O(C6H4)]2E2 [E = S (1), Se (2), Te (3)] were prepared and structurally characterized by multinuclear NMR (1H, 13C, 77Se or 125Te, as appropriate) and ESI+ mass spectrometry. The oxidation reaction of the diorganodichalcogenides 2 and 3 with SO2Cl2 in a 1:1 molar ratio under anhydrous conditions led to the phenoxaselenine 10,10-dichloride (5) and the organotellurium trichloride, [(C6H5)O(C6H4)]TeCl3 (4), respectively. When the oxidation reaction mixture was subjected to hydrolysis with a KOH solution, in case of the selenium containing species a mixture of phenoxaselenine 10-oxide (7) and phenoxaselenine (9) was formed. The crystal and molecular structures of compounds 2 - 5 and 7 were determined by single-crystal X-ray diffraction. The voltammetric behavior of 2 and 3 during oxidation was investigated in MeCN solutions.
17. Silver(I) complexes of a new multidentate macrocyclic ligand with N/S/Se donor atoms.
R. A. Popa, A. Silvestru, A. Pop, Polyhedron 110 (2016) 197-202. DOI: 10.1016/j.poly.2016.02.045.
The 28 membered macrocyclic ligand 1 containing S, Se and N donor atoms was obtained by the 2 + 2 condensation reaction between bis(o-formylphenyl)selenide and bis(2-aminoethyl)sulfane. The reaction between 1 and AgX [X = OSO2CF3- (2), PF6- (3) and BF4- (4)] resulted in silver(I) complexes of ligand 1. The NMR spectra suggest a mixture of two isomers of ligand 1 in chlorinated solvents, while for the metal complexes the spectra indicate only one species in solution. The molecular structures of 1 and the silver complexes 2 and 3·&2CHCl3 were determined by single-crystal X-ray diffraction. A distorted octahedral environment was observed for the silver atom in both complexes.
18. Organoselenium compounds with (N,C,N) pincer ligands. Structure and chemical behaviour.
A. Pop, A. Silvestru, J. Organomet. Chem. under evaluation.
The new diorganodiselenides [{2,6-(MeNCH2CH2)2NCH2}C6H3]2Se2 (1) and [{2,6-(OCH2CH2)2NCH2}C6H3]2Se2 (2) were obtained as brown oils by using the ortho lithiation route. The organoselenium(II) chlorides of type RSeCl were prepared by the cleavage of the Se-Se bond in 1, 2 and [2,6-(Me2NCH2)C6H3]2Se2 (3) with SO2Cl2. The organoselenium chlorides 4 - 6 are ionic species containing [RSe]+ cations. In reaction with AuCl(tht) these organoselenium chlorides was observed to transfer the halogen to gold, thus resulting in ionic organoselenium dichloroaurates of type [RSe]+[AuCl2]- (R = 2,6-(MeNCH2CH2)2NCH2}C6H3 (7), 2,6-(OCH2CH2)2NCH2}C6H3 (8) and 2,6-(Me2NCH2)C6H3 (9). The reactions between the lithium organoselenolates RSeLi and AuCl(PPh3) result in gold complexes of type RSeAu(PPh3). The molecular structures of [{2,6-(OCH2CH2)2NCH2]C6H4}Se]+Cl- (5) and [{2,6-(Me2NCH2)2C6H3}Se]+AuCl2- (9) were determined by single-crystal X-ray diffraction.
19. New hypervalent organoselenium compounds with O→Se intramolecular interactions.
R. A. Popa, A. Pop, C. Silvestru, A. Silvestru, Rev. Roum. Chim. 61 (2016) 495-501.
New organoselenium(II) compounds containing the 2-(O=CH)C6H4Se fragment are described. The reaction between [2-(O=CH)C6H4]2Se2 and [R2P(S)S]2 in a 1:1 molar ratio resulted in the formation of the organoselenium(II) derivatives [2-(O=CH)C6H4]SeSP(S)R2 [R = Ph (1), OPri (2)]. Compound 1 was obtained also by reacting [2-(O=CH)C6H4]SeCl (3) with NH4[S2PPh2] in a 1:1 molar ratio. While compound 1 was isolated as a solid, crystalline product, compound 2 could be evidenced only in equilibrium with the starting materials. The obtained products were investigated by multinuclear NMR (1H, 13C, 31P, 77Se). Compound 1 was also characterized by IR spectroscopy. The crystal and molecular structures of compounds 1 and 3 were determined by single-crystal X-ray diffraction.
20. New diorganochalcogen compounds based on the 2-(Me2NCH2)C6H4 group.
B. Danciu, R. A. Popa, A. Pop, V. Zaharia, C. Silvestru and A. Silvestru, Studia Universitatis Babes-Bolyai, Seria Chemia, LXI (2016) 19-28.
Heteroleptic diorganochalcogen compounds of type R[2-(Me2NCH2)C6H4]E [R = (phtz)CH2, E = Se (1), S (2); R = (4-Cl-phtz)CH2, E = S (3); R = (pz)CH2CH2, E = Se (4)] were prepared by reacting 2-(Me2NCH2)C6H4ELi with the appropriate organic halide in a 1:1 molar ratio. The new compounds were investigated in solution by 1H, 13C and 77Se NMR where appropriate. For compounds 1 and 4 the crystal and molecular structures were determined by single-crystal X-ray diffraction.
Conferences attended in 2012-2016
- Group 16 (Se, Te) hypervalent compounds. Synthesis, structural diversity, chemical reactivity.
Anca Silvestru,
oral presentation at Technische Universitä Chemnitz (Nov. 2012, Chemnitz).
- Hypervalent organoselenium compounds. Synthesis, structural diversity, chemical reactivity.
Anca Silvestru,
invited lecture at Rennes (Apr. 2013, Rennes).
- Diorganochalcogen(II) species bearing alkoxo functionalities and their coordination behavior.
Raluca Mitea, Anca Silvestru,
poster presentation at ICOMC 2012 (2 - 7 Sep. 2012, Lisabona).
- New diorganoselenium(II) ionic species.
Alexandra Pop, Anca Silvestru, Cristian Silvestru,
poster presentation at ICOMC 2012 (2 - 7 Sep. 2012, Lisabona).
- Organometallic species - potential precursors for group 12 metal chalcogenides.
Ana Maria Toma, Anca Silvestru,
poster presentation at EUCOMC 2013 (30 Jun. - 04 Jul. 2013, St. Andrews).
-
Organochalcogen compounds and their behaviour towards group 12 metals.
Mélanie Foret Jacquard, Cristina Coza and Răzvan I. Şuteu,
oral presentation at 10th International Conference "Students for Students" (10-14 Apr. 2013, Cluj-Napoca).
- Hypervalent organometallic compounds with relevance for nanomaterials and biology.
Anca Silvestru,
invited lecture at Zilele Academice Timisene, Academia Romana, Filiala Timisoara (26-27 May 2014, Timisoara).
- Organotellurium compounds - potential precursors for nanomaterials.
Nora Chiorean, Denes Eleonora, Anca Silvestru,
poster presentation at Zilele Academice Timisene, Academia Romana, Filiala Timisoara (26-27 May 2014, Timisoara).
- Organoselenium compounds with N,C,N-pincer ligands. Structure and chemical behavior.
Alexandra Pop, Roxana Popa, Anca Silvestru,
poster presentation at 5th EUCHEMS Chemistry Conference (31. Aug. - 04 Sep. 2014, Istanbul).
- New hypervalent tin(IV) pseudo-halides. Synthesis and structural characterization.
Cristina Coza, Adina Stegarescu, Anca Silvestru,
poster presentation at A-XXXIII-a Conferinta Nationala de Chimie (01 - 03 oct. 2014, Caciulata).
- Thermal decomposition studies on iron(III) mercapto-thiadiazol-thiocarboxylato precursors for iron-based materials.
Monica M. Venter, Vasile N. Bercean, Firuta Goga, Mircea Nasui,
poster presentation at A-XXXIII-a Conferinta Nationala de Chimie (01 - 03 oct. 2014, Caciulata).
- From diorganodichalcogenides to heterocycles.
Anca Silvestru,
oral communication at TRAMECH VIII, Antalya, Turkey, Nov. 2015.
- New multidentate macrocyclic ligands containing Se/S/N donor atoms and their late d-metal complexes.
Alexandra Pop,
poster presentation at EuCOMC XXI International Conference on Organometallic Chemistry, Bratislava, Slovakia, July 2015.
- New organotellurium compounds with 2-(Me2NCH2)C6H4 groups.
Denes Eleonora, poster presentation at 12th International Conference Students for Students, Cluj-Napoca, Romania, April 2015.
- Synthetic strategies for zinc- and copper-zinc ferrite preparation using oxalate precursors.
M. Venter, I. E. Nagy, A. L. Erdei-Ardeleanu, F. Goga, M. Nasui,
poster presentation at A XXXIV-a Conferinţă Naţională de Chimie, cu participare internaţională, 04-07.10. 2016, Călimăneşti-Căciulata, Romania.
- Solution behaviour of hypervalent organotellurium compounds.
Denes Eleonora, Anca Beleagă, Anca Silvestru,
poster presentation at The Central and Eastern European Bruker Users' Meeting, CEUM, 18 - 20.09.2016, Sofia, Bulgaria.
- Group 12 Metal Complexes with Organoselenium Ligands.
Alexandra Pop, Anca Silvestru, Clement Bellini, Yann Sarazin,
poster presentation at The 13th International Conference on the Chemistry of Selenium and Tellurium, ICCST 13, Mai, 23 – 27, 2016, Gifu, Japan.
- Hypervalent Organoselenium Compounds with Alkoxo Functionalities and their Coordination Behavior.
Raluca Mitea, Alexandra Pop, Anca Silvestru,
oral presentation at The 13th International Conference on the Chemistry of Selenium and Tellurium, ICCST 13, Mai, 23 – 27, 2016, Gifu, Japan.
- New multidentate macrocyclic ligands containing N/O or N/S donor atoms and their metal complexes.
Alexandra Pop, Anca Silvestru, Vito Lippolis,
poster presentation at The 5th Workshop of SeS Redox and Catalysis (WSeS-5), Mai, 21 – 22, 2016, Hyratsuka, Japan.
- Alexandra Pop, Studies on synthesis, structure and chemical reactivity of some new organo-chalcogen compounds.
PhD Thesis defended in 2012.
- Dorel Oltean, Complecsi ai unor metale tranzitionale din blocul d cu potentiala activitate catalitica.
PhD Thesis will be defended in 2013.
- Raluca Mitea, Complecsi ai metalelor tranzitionale cu liganzi cu atomi donori din grupele 15 si/sau 16.
PhD Thesis will be defended in 2013.
- Ana Maria Toma, Hypervalent compounds of group 15 metals (Sb, Bi) with metal - chalcogen bonds, precursors for nanomaterials.
PhD Thesis will be defended in 2014/2015.
- Coza Cristina, Compusi staniu(IV)-organici hipervalenti - precursori pentru materiale electronice.[Hypervalent tin(IV) compounds - precursors for electronic materials]
BSc Thesis, July 2013.
- Răzvan I. Şuteu, Complecsi cu legatura Cd-Se - precursori pentru materiale electronice. [Cadmium complexes with Cd-Se bonds - precursors for electronic materials].
BSc thesis, July 2013.