Abstract
Ferulic acid decarboxylase from Saccharomyces cerevisiae (ScFDC1) bears a novel cofactor in the form of a prenylated flavin mononucleotide (prFMN), which is a prerequisite for its unprecedented and, up to this point, unique in nature, 1,3-dipolar cycloaddition mechanism. The enzyme’s high tolerance towards non-natural substrates renders FDC1 an ideal biocatalyst for decarboxylation reactions yielding commercially valuable styrenes from cinamic acid derivatives. Moreover, rational design driven expansion of its substrate scope can be achieved through site-directed mutagenesis, replacing high-volume amino acids in the vicinity of the active site with their smaller counterparts – thus allowing bulkier or non-linear compounds to access the substrate-binding pocket.
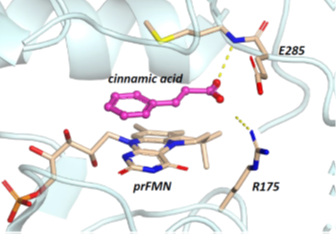
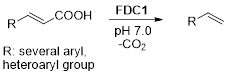
ScFDC1 mediated decarboxylation of cinnamic acid derivatives, involving the prFMN cofactor and catalytically essential E285 and R175 active site residues.
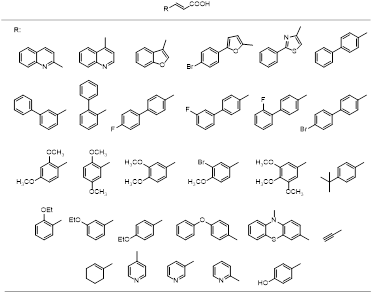
Substrate library for testing ScFDC1 activity